Mechanosensing
Cells explore their environment by sensing and responding to mechanical forces. Many fundamental cellular processes, such as cell migration, differentiation, and homeostasis, take advantage of this sensing mechanism. At molecular level mechanosensing is mainly driven by mechanically active proteins. These proteins are able to sense and respond to forces by, e.g., undergoing conformational changes, exposing cryptic binding sites, or even by becoming more tightly bound to one another. In humans, defective responses to forces are known to cause a plethora of pathological conditions, including cardiac failure, pulmonary injury and are also linked to cancer. Microorganisms also take advantage of mechano-active proteins and proteins complexes. Employing single-molecule force spectroscopy with an atomic force microscope (AFM) and steered molecular dynamics (SMD) simulations we have investigated force propagation pathways through a mechanically active protein complexes.Spotlight: Cells Get Sticky with Calcium (Apr 2008)
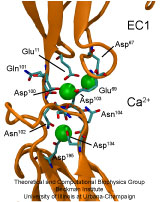
image size:
97.1KB
made with VMD
Adhesion between human cells organizes our body into its organs and parts. The adhesion comes about through an intricate system of molecules that perform their task in a highly selective manner such that the body's different types of cells will find the right cells and stick to them. This selectivity leads to tissue differentiation and the organization of organs as complicated as the brain. Cadherin proteins form a particular family of such adhesion molecules. Interestingly, they glue cells together only in the presence of calcium. Some members of the cadherin family of proteins are also involved in the transduction of sound and cadherin mutants are known to cause hereditary deafness (see the April 2005 highlight, "Hearing: Turning Sound into Voltage"). How cadherins selectively bind to each other and the role of calcium was not well understood, but now molecular dynamics simulations have offered magnificent insight into calcium's role as recently reported. The simulations took advantage of parallel supercomputers and NAMD's ability to harness their power. The simulations revealed that in the absence of calcium cadherins stick out of cell surfaces like ends of loose rope; in the presence of calcium cadherin molecules turn into stiff hooks that link cells together. The calcium-induced links can withstand the strong mechanical forces that arise between cells much larger than cadherin (more on our cadherin website).



