The Titin/Telethonin Complex
A Molecular Bungee Cord
In the extreme sport of bungee jumping, a daring athlete leaps from a great height and free-falls while a tethered cord tightens and stretches to absorb the energy from the descent. The bungee cord protects the jumper from serious injury, because its elasticity allows it to extend and provide a cushioning force that opposes gravity during the fall. Amazingly, nature also uses elasticity to dampen biological forces at the molecular level, such as during extension of a muscle fiber under stress. The molecular bungee cord that serves this purpose in the human muscle fiber is the protein titin, which functions to protect muscle fibers from damage due to overstretching.
The Muscle Fiber
Striated muscle, such as a skeletal or cardiac muscle, derives its name from the appearance of lines or grooves along its surface. These lines are actually a complex of elongated fibers arranged in parallel order and held together with connective tissue. Muscle fibers are typically 2 to 3 cm long and 100 µm in diameter. Fibers are built from smaller units called myofibrils (1-2 µm in diameter), which primarily contain thick myosin filaments (12-18 nm diameter) and thin actin filaments (5-8 nm diameter). During muscle contraction, metabolism of ATP induces movement of cross bridges between thick and thin filaments, leading to a shortening of the muscle fiber.
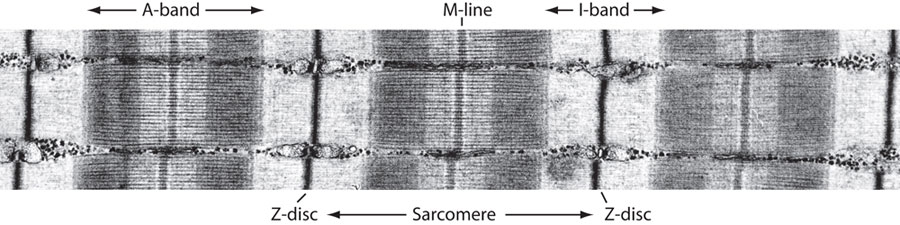
Electron microscope image of striated
muscle (courtesy Roger Craig, University of Massachusetts)
This
image represents multiple
myofibrils aligned in parallel, and the distinguishing features are
labeled. The A-band (dark band) consists mainly of thick myosin
filaments
(joined at the M-line), whereas the I-band (light band)
are composed of thin actin filaments. Each sarcomere is flanked
by a Z-disc region.
Viewed lengthwise, the myofibril is seen as a series of bands of alternating composition, separated by line-like regions. The A band is a set of stacked thick filaments centered on the M line, overlapped on both sides by thin filaments, which compose the I band. In between each I band, a dense vertical region called the Z line divides the myofibrils into a functional unit called the sarcomere, which is the smallest self-contained contractile unit (resting length 2 to 3 µm) of the striated muscle.
The Elasticity of Muscle
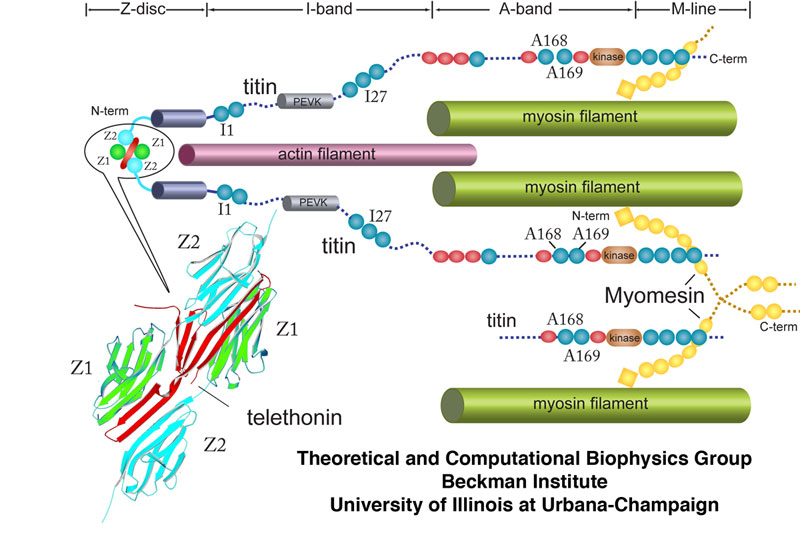
Schematic cross-section of the sarcomere showing major macroscopic components. In particular, the large protein titin is illustrated along with its various domains such as I1, I27, and A168-169. The Z-disc protein telethonin is also shown interacting with two separate titin molecules at their Z1 and Z2 domains.
An essential characteristic of striated muscle is its elasticity, a mechanical property necessary for the reversible contractile and stretching movement of sarcomere. This elasticity permits myofibrils to be stretched to twice their resting length without damaging their structure. The physiological sarcomere length ranges from 1.9 to 2.5 µm in cardiac muscle and 2.2 to 3.8 µm in soleus, a skeletal calf muscle. Investigations of myofibrils during contraction and relaxation suggest that neither actin nor myosin filaments confer the elasticity seen during force generation, since the length of both filaments appear unchanged during the contraction-extension cycle. Instead, the elastic property of sarcomere appears to arise from another set of muscular filaments, composed of the protein titin. It is the lengthening of titin molecules that generates the passive force which is crucial for maintaining the integrity of the sarcomere.
Titin is the largest protein identified in nature,
with a molecular weight of nearly ~4 MDa and a contour length
of ~1 µm (![]() ,
, ![]() ). Anchored at the sarcomeric Z and M
lines, a string-like titin molecule spans half of the sarcomere in
rigid contact with the thick filament along the A band, but flexible
along the I band.
). Anchored at the sarcomeric Z and M
lines, a string-like titin molecule spans half of the sarcomere in
rigid contact with the thick filament along the A band, but flexible
along the I band.
Electron micrographs and gene sequence analysis
reveal that the
architecture of titin is built upon a linear assembly of 300
immunoglobulin-like
(Ig) or fibronectin III-like
(FN-III) domains,
as well as a few other unique domains such as PEVK regions (![]() ,
, ![]() ). These domains control the passive
elasticity of
sarcomere and regulate a number of tension-mediated activities. In the
A-band and
Z-line regions, titin intimately associates with proteins located in
the thick and thin filaments, respectively. The only part of titin
that appears to move freely is its I-band region, which provides the
major contribution to passive elastic properties for the sarcomere.
Titin I-band domains of variable composition, called isoforms, have
been found in different types of muscle tissue, indicating that the
specific distribution of domain types within the titin spring
determine its stiffness and extensibility.
). These domains control the passive
elasticity of
sarcomere and regulate a number of tension-mediated activities. In the
A-band and
Z-line regions, titin intimately associates with proteins located in
the thick and thin filaments, respectively. The only part of titin
that appears to move freely is its I-band region, which provides the
major contribution to passive elastic properties for the sarcomere.
Titin I-band domains of variable composition, called isoforms, have
been found in different types of muscle tissue, indicating that the
specific distribution of domain types within the titin spring
determine its stiffness and extensibility.
The mechanical properties of titin Ig domains have
been experimentally
studied using AFM and optical tweezers (![]() ,
, ![]() ,
, ![]() ) and corroborated
with MD simulations (
) and corroborated
with MD simulations (![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() ).
These studies on the elastic properties of titin modules have
demonstrated
that titin functions as a mechanical force bearing protein crucial for
the stability of the muscle fibers.
).
These studies on the elastic properties of titin modules have
demonstrated
that titin functions as a mechanical force bearing protein crucial for
the stability of the muscle fibers.
The Titin Z1Z2/Telethonin Complex
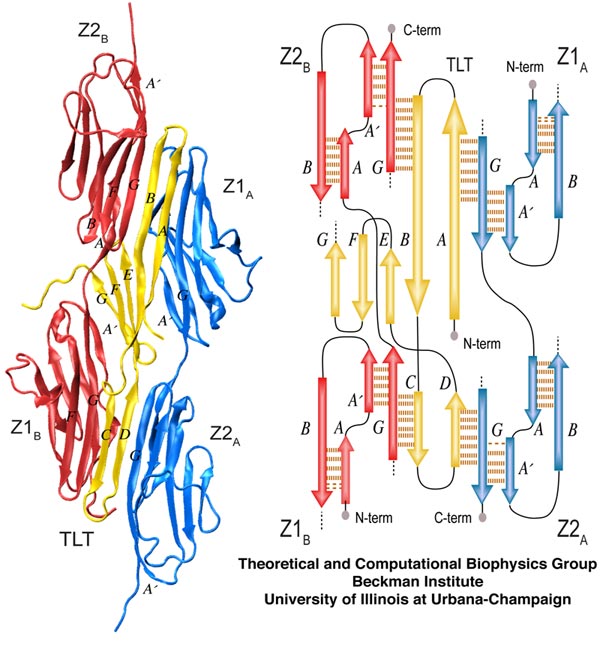
Overview of the Titin Z1Z2-Telethonin complex. The cartoon view on the left shows pairing of Z1Z2 dimers on each side of telethonin. The schematic on the right depicts the overall β-strand alignment and associated interstrand hydrogen bonds.
While the structural role of Ig and FN-III domains
near the center of titin molecule
have been studied extensively, the contribution that N- and C-terminal
titin
domains lend to the mechanical stability of titin is less well
understood. The N-terminal of titin interacts with several sarcomeric
proteins at the Z-disc. These interactions
provide structural support for the development and function of
myofibrils and also regulatory roles such as responding to
stretching forces developed in titin. Unique among them is the
structural protein telethonin, which is believed to anchor the ends of
two separate titin molecules to the Z-disc (![]() ,
, ![]() ).
).
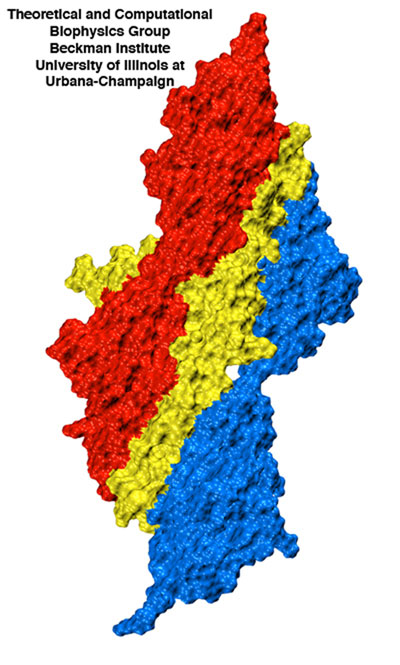
Surface view of the titin Z1Z2-telethonin complex illustrating the intimate contact between telethonin (yellow) and two titin molecules (red and blue).
Biochemical studies of telethonin
have shown that its presence in conjunction with the two N-terminal
titin modules
Z1 and Z2 is required for initial muscle growth(![]() ), and point mutations to telethonin
resulting in premature stop codons have been correlated to a form of
limb girdle muscular distrophy (type 2A)(
), and point mutations to telethonin
resulting in premature stop codons have been correlated to a form of
limb girdle muscular distrophy (type 2A)(![]() ), suggesting that telethonin
plays a major role in the initial assembly and stabilization of
N-terminal titin at the Z-disc. Destabilization of the Z-disc region of
titin has also been associated with dilated cardiomyopathy(
), suggesting that telethonin
plays a major role in the initial assembly and stabilization of
N-terminal titin at the Z-disc. Destabilization of the Z-disc region of
titin has also been associated with dilated cardiomyopathy(![]() ), indicating that disruption of the
telethonin-titin Z1Z2 interface may be a direct cause of this pathology(
), indicating that disruption of the
telethonin-titin Z1Z2 interface may be a direct cause of this pathology(![]() ).
).
The recently resolved crystal structure of
telethonin in complex with titin Z1 and Z2
reveals a novel binding motif between Ig domains and a
ligand (![]() ). Unlike typical ligand-binding through
insertion of the ligand into a receptor binding pocket, the receptor
Z1Z2 domains of two antiparallel
titin N-termini are surprisingly joined together by an N-terminal
fragment of the ligand telethonin through β-strand
cross-linking, a structural motif that also appears in
pathological fibril formation (amyloidoses). Therefore, telethonin's
function in stabilizing the titin Z1 and Z2 domains may lie in the fact
that it joins two titin molecules together in a gluelike fashion.
). Unlike typical ligand-binding through
insertion of the ligand into a receptor binding pocket, the receptor
Z1Z2 domains of two antiparallel
titin N-termini are surprisingly joined together by an N-terminal
fragment of the ligand telethonin through β-strand
cross-linking, a structural motif that also appears in
pathological fibril formation (amyloidoses). Therefore, telethonin's
function in stabilizing the titin Z1 and Z2 domains may lie in the fact
that it joins two titin molecules together in a gluelike fashion.
Experimental and computational studies (mentioned above) have shown previously that single titin Ig-domains such as I27 are well suited for bearing a mechanical force. The discovery of titin Z1Z2 Ig-domains in complex with telethonin naturally poses questions: Are the force bearing properties of these Ig-domains significantly altered by their interaction with telethonin? What are the mechanical features of force bearing protein complexes, as opposed to individual proteins, and why are they important? The titin Z1Z2/telethonin complex, therefore, represents a tremendous opportunity to study two biological phenomena at once: 1) a multiprotein complex essential for proper muscle function, and 2) the role that intermolecular β-strand crosslinking plays in adhesion of healthy proteins, which itself is of great interest in light of the attention given lately to amyloidoses such as Alzheimer's, Parkinson, and Huntington's disease.
Probing the Strength of A Molecular Glue

Forced unbinding of the titin Z1Z2-telethonin complex. Shown in the plot is the distance between two stretched titin Z2 α-carbon termini during constant force SMD simulations at forces of 750pN (dk blue), 1000pN (green), 1200pN (red), and 1500pN (black), respectively. Snapshots taken from the unbinding trajectory of the 1200pN simulation represent (A) the initial equilibrated structure, (B) and (C) the transition events where both titin Z2 domains separate from telethonin, and (D) the subsequent unraveling of one Z2 domain once freed from telethonin.
To address the questions above, we have studied the
mechanical design of
the Z1Z2-telethonin complex and its individual components by comparing
their
mechanical responses through so-called steered
molecular dynamics (SMD) simulations (![]() ) that stretch
the two systems by pulling their termini diametrically apart. It turns
out that the
mechanical stability of the complex is much stronger than individual
Ig domains (
) that stretch
the two systems by pulling their termini diametrically apart. It turns
out that the
mechanical stability of the complex is much stronger than individual
Ig domains (![]() ). Destabilizing the complex by stretching
the titin Z2 terminal ends of the complex requires higher peak force
than that required for unfolding single titin Ig-domains such as I27.
). Destabilizing the complex by stretching
the titin Z2 terminal ends of the complex requires higher peak force
than that required for unfolding single titin Ig-domains such as I27.
Our simulations also reveal that the Z1Z2-telethonin complex exhibits significantly higher resistance to mechanical stretching forces than titin Z1Z2 alone, suggesting that telethonin plays a role in anchoring titin to the sarcomeric Z-disc. Whereas previous studies have revealed that the unraveling of the β-strands for titin Ig-domains constitutes the dominant unfolding barrier, in the case of Z1Z2 in complex with telethonin, the force peak observed corresponded to a detachment of one virtually intact Z2 domain from a β-strands of telethonin.
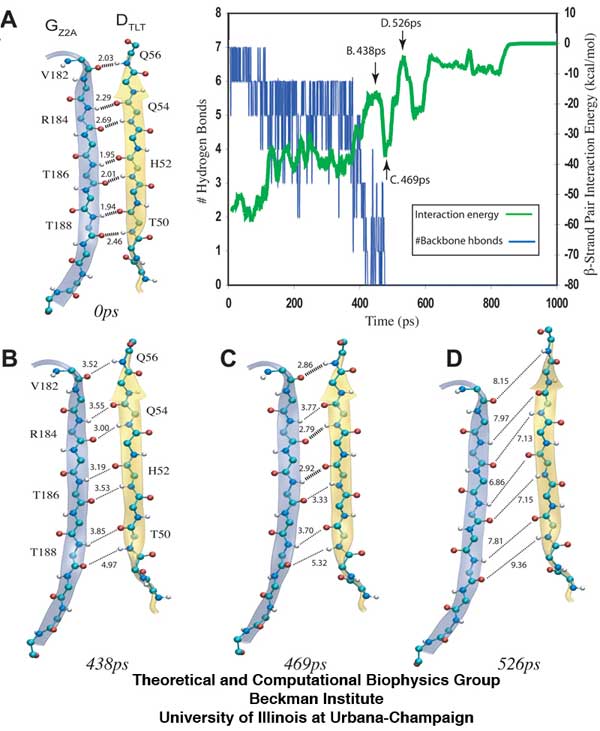
The separation of β-sheets between titin Z2 and telethonin during the 1200pN SMD simulation reveals that a series of hydrogen bonds are responsible for gluing the two proteins together. The snapshots (A-D) show the distance between hydrogen bonding pairs increasing as force is applied over time. The inset plot reveals an increase in energetic instability (green) as the number of stable hydrogen bonds decrease (blue).
This discovery sheds light on a key structural strategy that the titin Z1Z2-telethonin complex employs to yield extraordinary resistance to mechanical stress. The major force bearing component of this complex is an extensive intermolecular hydrogen bonding network formed across β-strands between telethonin and Z1Z2 domains, and not intramolecularly between termini β-strands of individual Z1 or Z2 domains. This shift to a stronger force bearing interface reduces the chance of unraveling the individual Ig-domains, thus stabilizing the complex. This example demonstrates how β-strand cross-linking via hydrogen bonds serves as an important mechanism, in effect a molecular glue, for augmenting the ability of protein complexes designed to resist mechanical stress.
We also examined the stability of the N-terminal (residues 1 to 89) telethonin "glue" molecule by itself when it is unbound to either titin Z1 or Z2. MD simulations revealed that β-sheet projections on telethonin twist, or fold in upon, themselves in the absence of Z1 and Z2. This suggests that during the initial stages of muscle growth, titin assembles first in order to provide a favorable interface for telethonin binding. In essence, the components that need to be joined together (two titin molecules) are staged into place before their molecular glue is added.
Beyond our primary conclusion that telethonin may
serve as a titin anchor, our simulations reveal also a fundamental
architectural element of living cells, namely, how cells glue their
components together yielding strong mechanical connections. The
structural motif employed by telethonin in this respect, namely,
cross-linking β-strands, has also been implicated in fibril
formation (![]() ) and it is important to take note of such
potential pathological side effects of telethonin's motif. Clearly,
the deployment of telethonin as a powerful glue needs to be carefully
controlled in the
cell and future work will likely focus on the control mechanisms
used in focusing telethonin and similar other, yet unknown, mechanical
proteins
on gluing the right protein components, and not the wrong ones,
avoiding in particular, self-aggregation.
) and it is important to take note of such
potential pathological side effects of telethonin's motif. Clearly,
the deployment of telethonin as a powerful glue needs to be carefully
controlled in the
cell and future work will likely focus on the control mechanisms
used in focusing telethonin and similar other, yet unknown, mechanical
proteins
on gluing the right protein components, and not the wrong ones,
avoiding in particular, self-aggregation.
- Click here for a movie (mpeg, 2.9M) of the unfolding trajectory of titin Z1Z2 under 750pN constant force.
- Click here for a movie (mpeg, 3.6M) showing an unfolding trajectory when a 1200pN force is used to pull both C-terminal titin Z2 domains of the titin Z1Z2-telethonin complex.
- Click here for a movie (mpeg, 6.7M) showing equilibration of telethonin and folding in of peripheral β-hairpins (19ns).
Publications and Related Commentary
Mechanical strength of the titin Z1Z2/telethonin complex. Eric H. Lee, Mu Gao, Nikos Pinotsis, Matthias Wilmanns, and Klaus Schulten. Structure, 14:497-509, 2006.
Also see a commentary of our work in the journal Structure
Molecular
mechanisms of cellular mechanics.
Mu Gao, Marcos Sotomayor, Elizabeth Villa, Eric Lee, and Klaus
Schulten. Physical Chemistry - Chemical Physics, 8:3692-3706,
2006.
Secondary
and tertiary structure elasticity of titin Z1Z2 and a
titin chain model.
Eric H. Lee, Jen Hsin, Olga Mayans, and Klaus Schulten. Biophysical
Journal, 93:1719-1735, 2007.
Investigators
- Eric H. Lee
- Mu Gao
- Klaus Schulten
- Mattias Wilmanns (EMBL Hamburg)
- Nikos Pinotsis (EMBL Hamburg)
- Olga Mayans (University of Basel)
Related TCB Group Projects
Page created and maintained by Eric H. Lee.
