Kinesin
Kinesin is the founding member of a superfamily of microtubule-based ATPase motors that perform force-generating tasks such as organelle transport and chromosome segregation. The recent crystal structure of a kinesin-related motor protein with the name 'nonclaret disjunctional' (ncd) is almost identical to kinesin [Kull et al. and Sablin et al., Nature 380 (1996)], although the two proteins move in opposite directions on microtubules. The conformational differences between ADP and ATP-bound states and the structural basis for the directionality of the movement of these motors is unknown. Recent electron microscopy experiments have shown that the orientation of the kinesin motor domain bound to a microtubule is nucleotide-dependent. We modelled the ATP-bound protein and to identified conformational differences relative to the ADP-bound state which can be attributed to a force-producing power stroke.
Comparison of the head domains of the microtubule-based motors ncd (red) and kinesin (blue) with bound ADP (orange) and Mg++ ion (green). The crystal structures, courtesy Ron Vale and Robert Fletterick, are visualized with the program VMD.
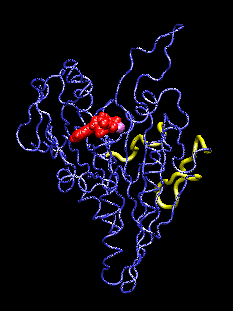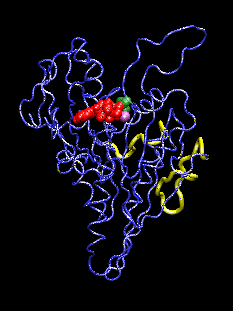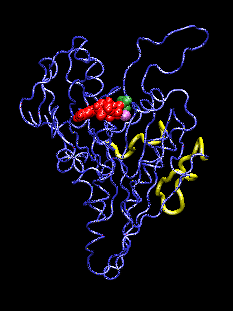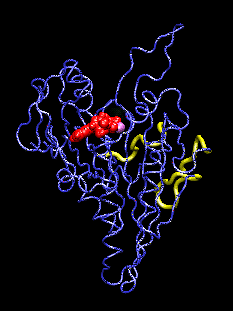
This movie (animated gif format) shows conformational changes induced in the kinesin structure (blue) by the additional gamma phosphate (green) of ATP. The shown conformation oscillates between model structures of ADP-bound and ATP-bound kinesin which have two putative microtubule binding regions (yellow) superimposed. ADP and the associated Mg ion are shown in red and purple, respectively. The movements visualized with the program VMD.
Click here for a larger (2.1M) version.
Simulated Annealing Simulations of Kinesin
The discovery of the crystal structure of the kinesin motor domain has made it possible to study kinesin's dynamics by computer simulations. The mentioned structure corresponds to ADP-bound kinesin at pH 4.6. To date, no structure of any ATP-bound kinesin has been solved. To model pH- and nucleotide-dependent changes in the kinesin structure, we carried out conformational searches by simulated annealing, using inter-atomic distance constraints derived from mutagenesis data and from structural comparisons with the kinesin-like motor ncd, G proteins, and the actin-based motor myosin. The resulting structures suggest a three-step scenario for the conversion of ATP hydrolysis into action: (1) The sensing mechanism for the additional gamma phosphate of ATP involves two key residues: Gly234 (corresponding to Gly466 in myosin) and Ser202 (Ser243 in myosin). (2) This step is followed by an observed transfer of the conformational changes to two putative microtubule binding sites, stabilized by a salt-bridge switch (residues 96/231/190). (3) We project changes in protein-microtubule interactions to result in a 11-degree rotation of the ATP-kinesin head relative to ADP-kinesin:
 Visit the updated version of this page at Willy Wriggers' new kinesin site.
Visit the updated version of this page at Willy Wriggers' new kinesin site.
References
If you use diagrams or material from this site, I ask that you cite the home page and author, or the appropriate source publication in your work. Copyright 1998-1999. All rights reserved.




