Highlights of our Work
2025 | 2024 | 2023 | 2022 | 2021 | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 | 2014 | 2013 | 2012 | 2011 | 2010 | 2009 | 2008 | 2007 | 2006 | 2005 | 2004 | 2003 | 2002 | 2001
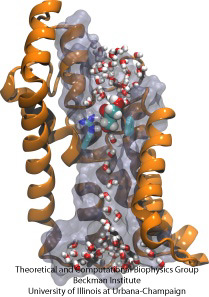
Biological cells protect their interior through their cellular membranes, yet rely on import of nutrients. They have evolved for this import fast conduction channels that include reliable checkpoints distinguishing desirable and undesirable compounds. A checkpoint puts up a veritable obstacle course that only the right compounds can pass quickly. Understanding the channel design is difficult due to lack of detailed experimental data on nutrient dynamics. Presently, the most detailed information comes from viewing channel dynamics computationally, starting from static crystallographic structures. A recent study investigated how glycerols, small nutrient molecules needed by some bacteria, pass through checkpoints realized through the membrane protein aquaporin (see also highlights Gas Molecules Commute into Cell - Mar 2007, Aquaporin and the Cambridge Five - Oct 2006, Cellular Faucets - Feb 2006). Aquaporin furnishes four parallel channels that were monitored computationally using NAMD and a novel algorithm that explores the channel energetics quickly enough to be methodologicaly feasible on today's computers. The results show how the physical characteristics of glycerol, for example the molecule's ability to form so-called hydrogen bonds, its electrical dipole moments, its diffusive mobility and intrinsic flexibility are probed along the channel, discriminating glycerol from other molecules. More on computational investigations of aquaporin here.