Highlights of our Work
2026 | 2025 | 2024 | 2023 | 2022 | 2021 | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 | 2014 | 2013 | 2012 | 2011 | 2010 | 2009 | 2008 | 2007 | 2006 | 2005 | 2004 | 2003 | 2002 | 2001
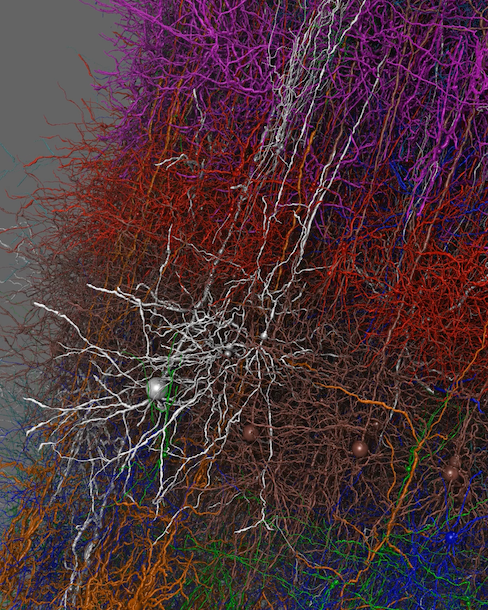
made with VND
Experimental techniques continue to provide neuroscientists with
structural and functional data on neurons at a rapidly increasing level
of detail; biophysically-detailed brain models and simulations can
provide integrated understanding of neuronal structures, connectivity,
and function. Visual
Neuronal Dynamics (VND) is
award-winning software for 3D visualization and exploration of
neuronal models and simulations. The software is produced by the Center,
in collaboration with Allen
Institute, and is compatible with the Brain Modeling Toolkit
(BMTK). In addition to readily visualizing models at multiple
scales, neuron
firings can be animated showing how "lightning storms" travel
through the brain. Advanced visualization techniques help the viewer
make sense of the dense neuronal thicket. The latest VND release includes flexible browsing and selection of neuron attributes, GUI improvements with information about neurons, and a refined line-only viewing mode.
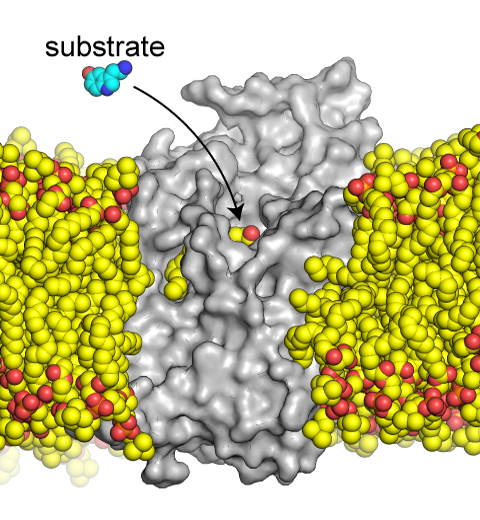
The serotonin transporter (SERT) is a protein in the cellular membrane of neurons that is responsible for the uptake of the neurotransmitter serotonin from the synapse back into the cell. Lipid-protein interactions have been demonstrated to regulate the transport properties of SERT. To understand the underlying mechanism, we collaborated with Eric Gouaux's lab at OHSU and investigated the binding of lipids to SERT using cryo-EM and molecular dynamics simulations. Our enhanced sampling simulations (performed in NAMD and analyzed in VMD) successfully captured a specific lipid that binds to a binding site in SERT. This site can also accommodate the substrate serotonin to trigger its transport. Therefore, binding of lipids to it is a putative mechanism for lipid-modulated function of SERT. For more details, see our recent publication in PNAS.
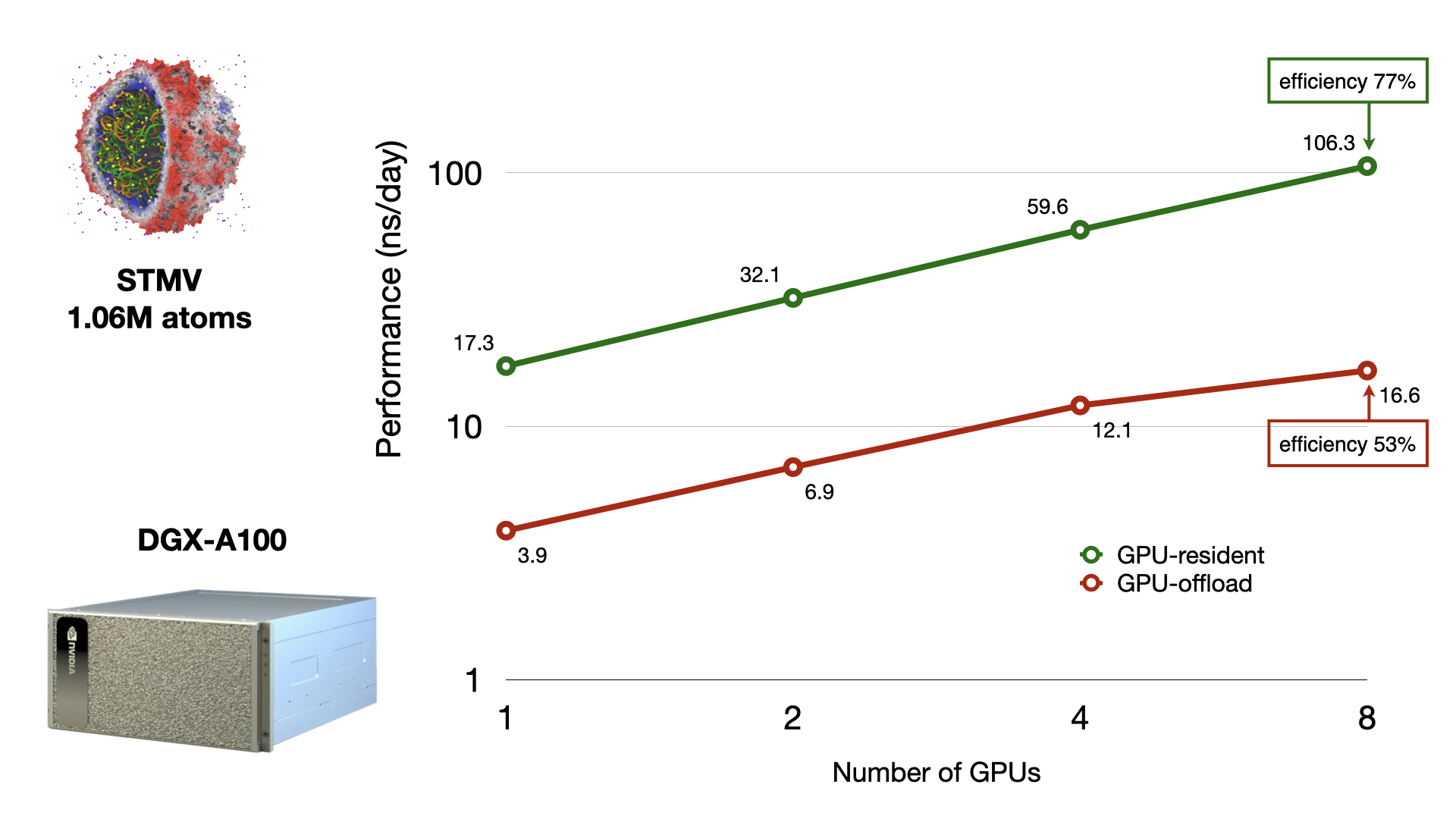
The new NAMD 3.0
provides a huge leap in performance through a new GPU-resident mode
— that is, all the calculations and data reside on the GPU.
This new GPU-resident mode more than doubles performance
of the program over the earlier versions.
Further work has made it possible to scale GPU-resident
NAMD
simulations across multiple GPUs.
The figure compares the scaling and performance of
NAMD
when simulating the 1M-atom STMV virus on a modern GPU machine
(NVIDIA DGX-A100).
The GPU-resident advancements benefit system sizes from 20k up to 20M atoms,
a range covering the most relevant sizes for biomedical investigation,
thereby accelerating simulation-based scientific discovery.
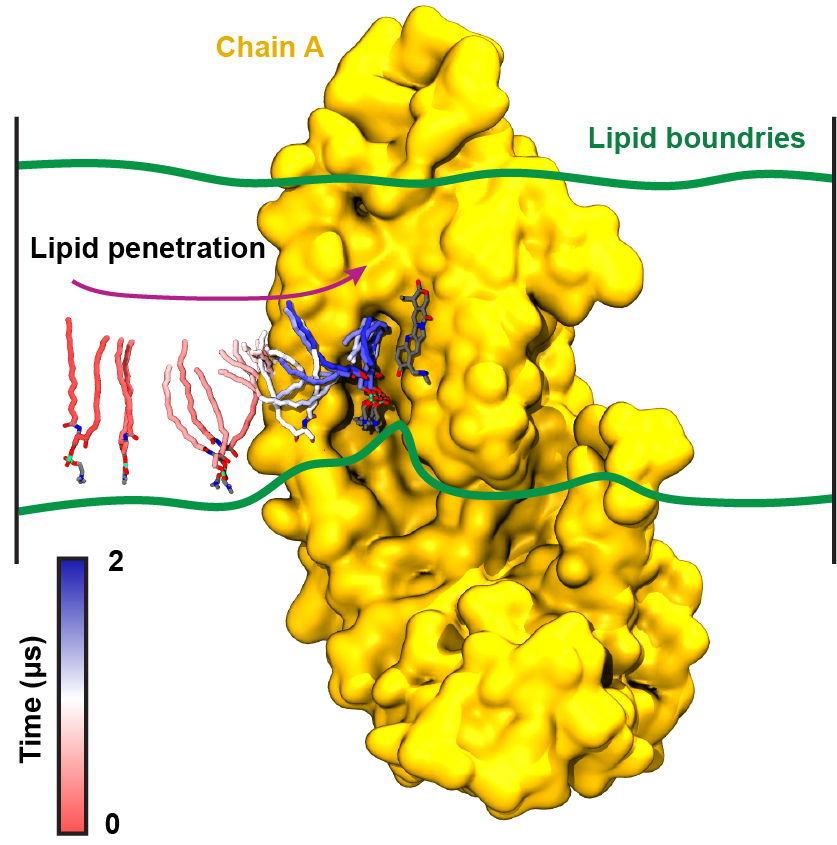
ABCG2, which is a crucial protein in development of multidrug resistance in cancer cells, is a transporter machine, acting like a vacuum cleaner in the membrane that removes a wide range of drugs from the cell. Given its heavily fatty environment, it's not surprising that its function is highly influenced by lipids and cholesterol. To shed light on the underlying mechanism, cryo-EM and functional assays in the Locher lab at ETH Zürich, were combined with computational microscopy using NAMD and VMD at the Center, to discover, for the first time, that lipids can enter the protein's binding pocket and directly interact with the bound drugs. More interestingly, cholesterol is found to guard the binding pocket's gates from other lipids, thus accelerating the transport function of the protein. Read more about the study in a recent PNAS paper.
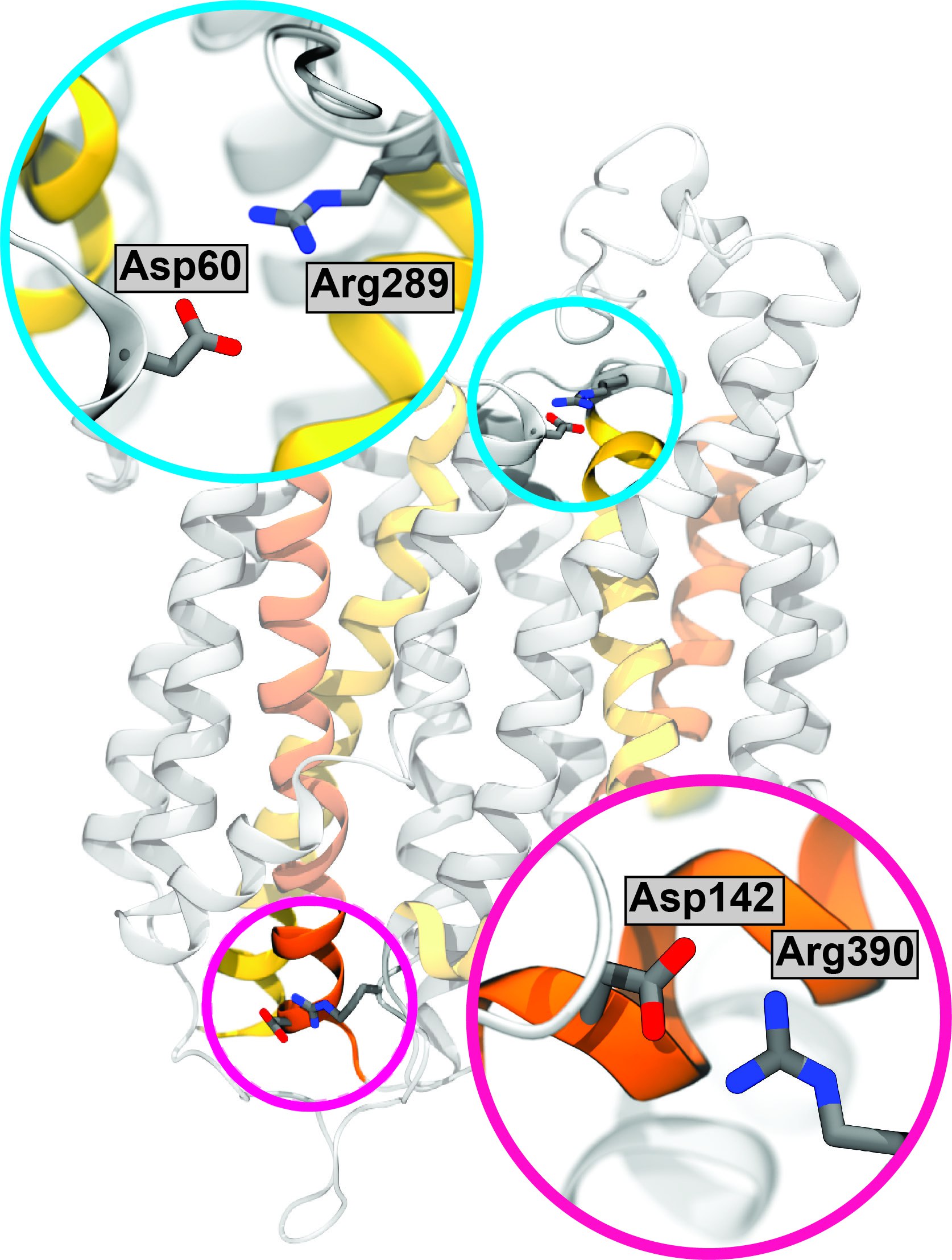
Spinster (Spns) transporters are critical for lipid transport across the cellular membrane, a process that regulates growth and migration of the cell in human body. Yet, the details of their molecular mechanism which could potentially help drug hunters, have remained elusive. Guided by DEER measurements and site-directed mutagenesis performed in Dr. Reza Dastvan's lab (St Louis University), molecular dynamics simulations by the Center's researchers were used to identify unknown conformational states of a Spns transporter (HnSpns). This systematic study reveals conserved proton-binding switches that modulate the structural transitions of the transporter, and thereby shed light on its proton-coupled mechanism. The simulations were performed with NAMD and analyzed by VMD. Read more in a Nature Communications.