Highlights of our Work
2025 | 2024 | 2023 | 2022 | 2021 | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 | 2014 | 2013 | 2012 | 2011 | 2010 | 2009 | 2008 | 2007 | 2006 | 2005 | 2004 | 2003 | 2002 | 2001
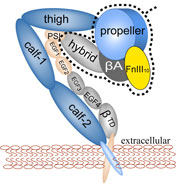
Mammalian cells adhere to each other forming tissues. The adhesion is due
to a network of proteins, so-called extracellular matrix proteins,
"gluing" the cells together. The cell membranes are too soft to provide
anchoring points for the extracellular matrix proteins; rather, the cells
furnish on their outer surface specialized hooks for anchoring the
extracellular matrix proteins. The hooks, in
the form of surface proteins, are linked directly through the membranes
to the intracellular cytoskeleton that stabilizes and shapes
cells. Integrins are an important family of such surface proteins that form
hooks specific for certain types of extracellular matrix proteins.
The hooks are flexible,
they can be open for contacts or closed, the switch being induced by signals
from inside or outside the cell through interactions with other proteins.
The interactions between integrins and extracellular matrix proteins
are rather complex, as the proteins are composed of many subunits;
fortunately, their
overall structures are presently being solved through crystallography.
In a recent
report a major component of an integrin and an extracellular matrix
protein have been investigated through molecular modeling using
NAMD, including steered molecular dynamics.
The study described in detail how the extracellular matrix protein induces
a transition in integrin, potentially strengthening its adhesion property.
See also previous highlights:
the May 2006 "Killer's Entry Route", Dec 2004 "Snap Fastener on Biological
Cells", Dec 2003 "Body's Glue", and Mar 2002 "Cells Sense Push and Pull".
More on modeling of extracellular matrix proteins and integrins can be found
here.