Highlights of our Work
2025 | 2024 | 2023 | 2022 | 2021 | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 | 2014 | 2013 | 2012 | 2011 | 2010 | 2009 | 2008 | 2007 | 2006 | 2005 | 2004 | 2003 | 2002 | 2001
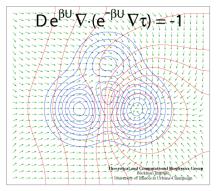
Processes in living cells are based on molecular transformations linking reactant and product states. To understand such processes, one needs to know the paths linking reactants and products. Brownian dynamics, as it occurs in cells at physiological temperature, finds any path, as long as it does exist, even if it may take billions of vibrational periods in a protein. But on the nanosecond time scale of computational modeling such paths are too rare to detect, even for the most powerful computers available today. Steered or interactive molecular dynamics , pioneered in our group, can accelerate motion along a reaction path within reach of available computational power, but only if the path is known beforehand. Principles for finding reaction paths are therefore the most desirable goals of theoretical biology. A recent study has formulated a new mathematical principle for determining reaction paths that is based on the mean first-passage time τ linking any intermediate state of the system to the product state. The principle has proven already its worth in describing pathways of light harvesting in photosynthesis, the process that energetically fuels life on earth.