Highlights of our Work
2025 | 2024 | 2023 | 2022 | 2021 | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 | 2014 | 2013 | 2012 | 2011 | 2010 | 2009 | 2008 | 2007 | 2006 | 2005 | 2004 | 2003 | 2002 | 2001
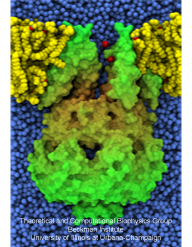
Bacteria employ membrane proteins as crucial safety valves that release water and small solutes
under challenging osmotic conditions (see the
May 2006 highlight,
"Electrical Safety Valve" and the Nov 2004
highlight, "Japanese Lantern Protein"). There are valves for balancing small pressure differences between
the inside and outside of bacterial cells, that open and close readily, but there are also
ones for protection against large pressure differences as a safety measure of last resort. The
valves for balancing small pressure differences, like the one shown in the figure, include
a filter that presumably keeps the most valuable molecules inside the cell interior, though
this is not understood yet in detail. To reveal the
function of such channels a combination of X-ray crystallography, physiological measurements, and molecular
dynamics simulations using NAMD has been employed.
Crystallography, in a prior study, captured the channel in a half-way open state. Now a team of physiologists and
modelers reported the details on valve opening and closing.
The experiments, using a pipette small enough to measure currents from a single channel, MscS, along with the simulations
revealed that the channel conducts both positive and negative ions when subjected to tension and voltage. The unprecedented
comparison of experimental and computational results open a new era of quantitative cell biology that borrows
successful research strategies from physics (more on our MscS website).