Highlights of our Work
2024 | 2023 | 2022 | 2021 | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 | 2014 | 2013 | 2012 | 2011 | 2010 | 2009 | 2008 | 2007 | 2006 | 2005 | 2004 | 2003 | 2002 | 2001
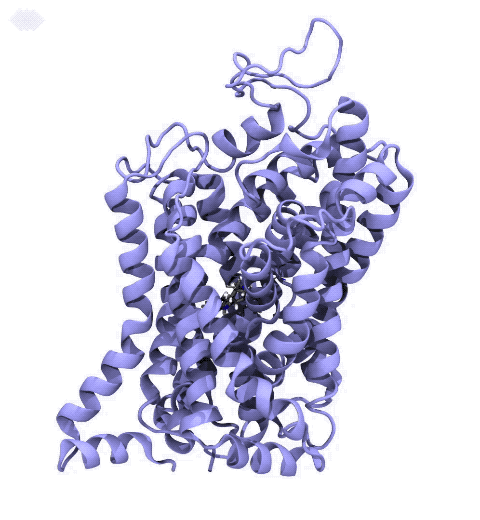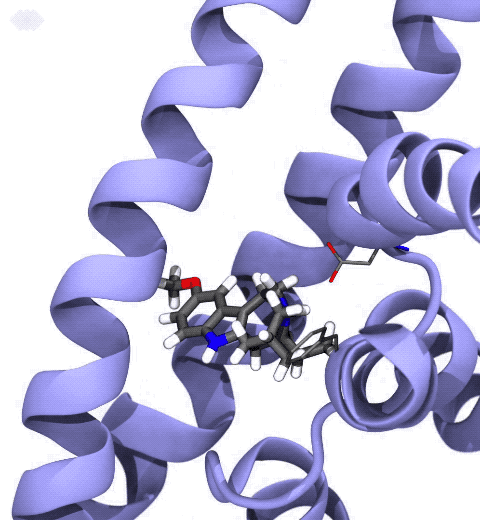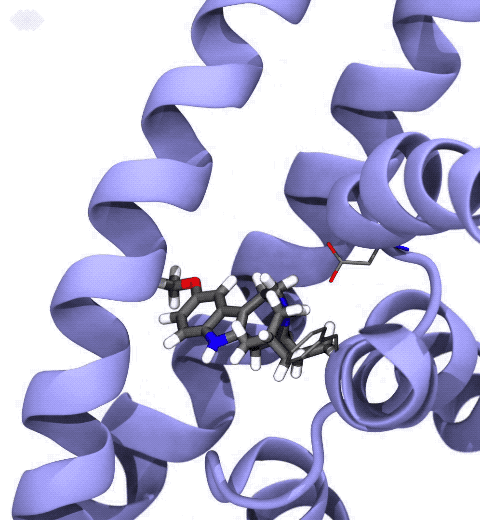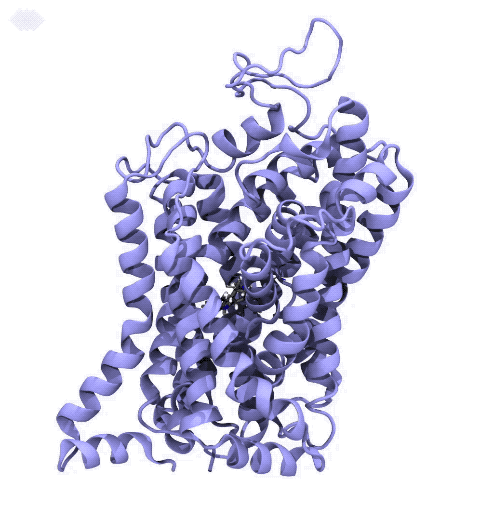
image size:
7.7MB
made with VMD
Neurotransmitters are chemical messengers diffusing across the synapse to propagate nerve impulses. The serotonin transporter (SERT) is a protein embedded in the cellular membrane of the pre-synaptic neuron, which recycles the neurotransmitter serotonin. It plays pivotal roles in modulating
behavior, rendering it a primary drug target for neuropsychiatric disease. Inhibition of the serotonin uptake by SERT contributes to the mechanism of clinically used antidepressants. To provide structural insights into the transport mechanism, our collaborators in the Gouaux lab (OHSU) captured a series of structures of SERT in multiple functional states using cryoEM. The structures were co-crystalized with an inhibitor, ibogaine, a hallucinogenic drug found in plants with anti-addictive potential, in the substrate-binding pocket. However, the exact binding poses of ibogaine in these structures remained ambiguous due to the limited resolution, prohibiting a direct interpretation of ibogaine's inhibition mechanism. To settle this problem, we developed a computational docking approach to systematically search for the most probable ibogaine binding poses, which best fit into the experimental density maps and exhibit considerable stability in the following by molecular dynamics simulations performed with NAMD. Read more in Nature.



