Highlights of our Work
2025 | 2024 | 2023 | 2022 | 2021 | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 | 2014 | 2013 | 2012 | 2011 | 2010 | 2009 | 2008 | 2007 | 2006 | 2005 | 2004 | 2003 | 2002 | 2001
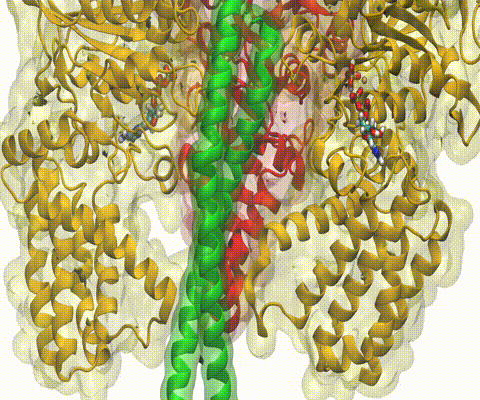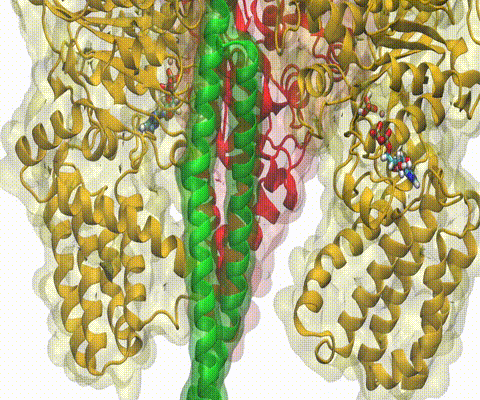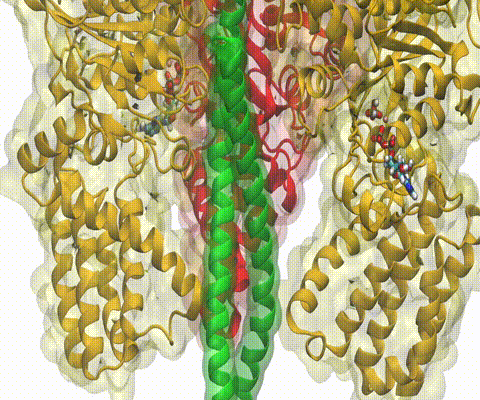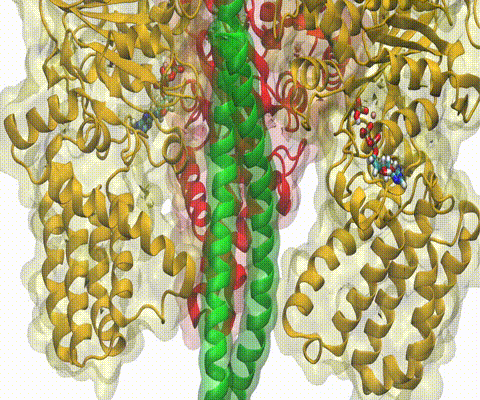
image size:
1.7MB
made with VMD
Living cells employ an intricate network of biochemical processes for the
conversion of solar energy or nutrients
into energy-rich Adenosine Tri Phosphate (ATP) molecules. From bacteria to
fungi, plants, and animals,
ATP serves as the universal energy currency of life, fueling the processes
cells need to survive and function.
Over the course of a day, an individual will typically use the equivalent of
his or her bodyweight in ATP; however, the human body carries only a small
amount of the molecule at any one time. That means cells must constantly
recycle or replenish the ATP molecules, relying on a highly efficient motor
protein called ATP synthase to do the job. Given its ubiquitous role in
photosynthesis and respiration, efficiency of the ATP synthase motor has been
a focus of intense biochemical investigations over the past three decades,
which included Boyer and Walker's Nobel Prize-winning contributions in 1997.
Yet, connection between the Chemistry of ATP dissociation and Physics of ATP
synthase motor-action remained elusive.
A recent study
based on molecular dynamics simulations with NAMD
reveals the working
principles of V-type ATP synthase in atomic resolution. Swiveling motions in
the protein ring were captured together with rubber band-like elasticity of
the motor's central stalk. This swiveling motion of the ring when paired with
the stalk absorbs about 75 percent of the energy released during ATP
hydrolysis, showcasing a molecular design that underlies the molecular motor's
remarkable energy-conversion efficiency.
More on ATPase here and
here.



