Highlights of our Work
2024 | 2023 | 2022 | 2021 | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 | 2014 | 2013 | 2012 | 2011 | 2010 | 2009 | 2008 | 2007 | 2006 | 2005 | 2004 | 2003 | 2002 | 2001
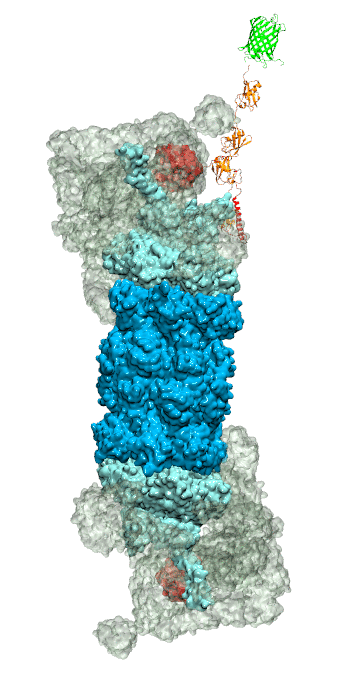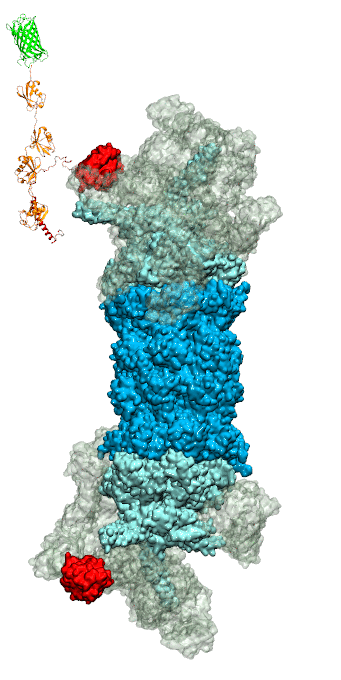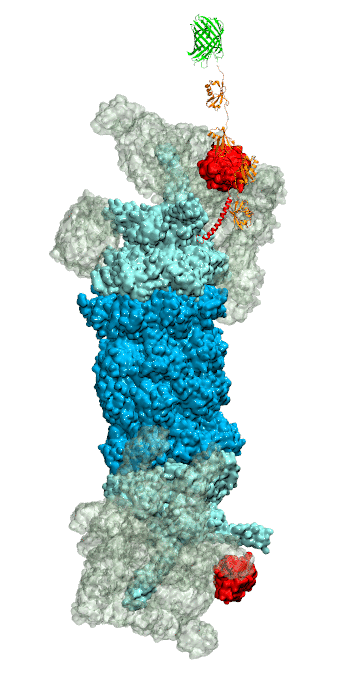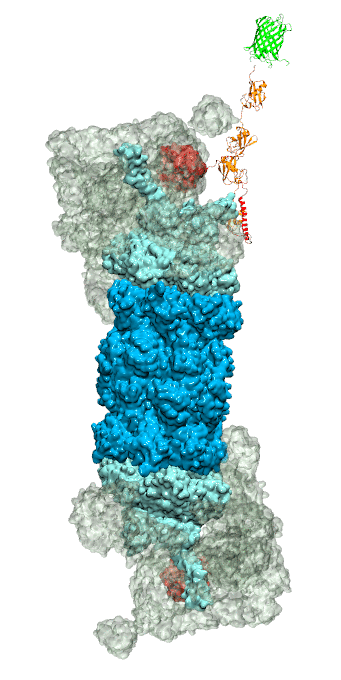
image size:
711.1KB
made with VMD
While waste recycling became popular in our daily life, living cells mastered
waste recycling of their protein content since their very beginning. Recycling
of unneeded protein molecules in cells is performed by a molecular machine
called 26S proteasome, which cuts these proteins into smaller pieces and
releases the pieces into the cell interior for reuse as building blocks for new
protein. Proteins that need to be recycled are usually those that are
misfolded. Proteins are recognized as such by the cells' so-called quality
control system. This system labels misfolded proteins by a tag made of
tetra-ubiquitin protein chains. The 26S proteasome machine recognizes and binds
to these tags via its subunit Rpn10. After Rpn10 binds to the tetra-ubiquitin
tag and pulls the protein close, the 26S proteasome unwinds the tagged protein
and cuts it into pieces. A recent
study, based on molecular dynamics simulations with NAMD, sheds light onto how 26S
proteasome and Rpn10 recognize the tetra-ubiquitin tag in three stages: In
stage 1 of the recognition process conserved complementary electrostatic
patterns of Rpn10 and ubiquitins guide protein association; stage 2 induces
refolding of Rpn10 and tetra-ubiquitin tag; stage 3 facilitates formation of
hydrophobic contacts between the tag and Rpn10. More information is available
on our 26S
proteasome website.



