Highlights of our Work
2025 | 2024 | 2023 | 2022 | 2021 | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 | 2014 | 2013 | 2012 | 2011 | 2010 | 2009 | 2008 | 2007 | 2006 | 2005 | 2004 | 2003 | 2002 | 2001
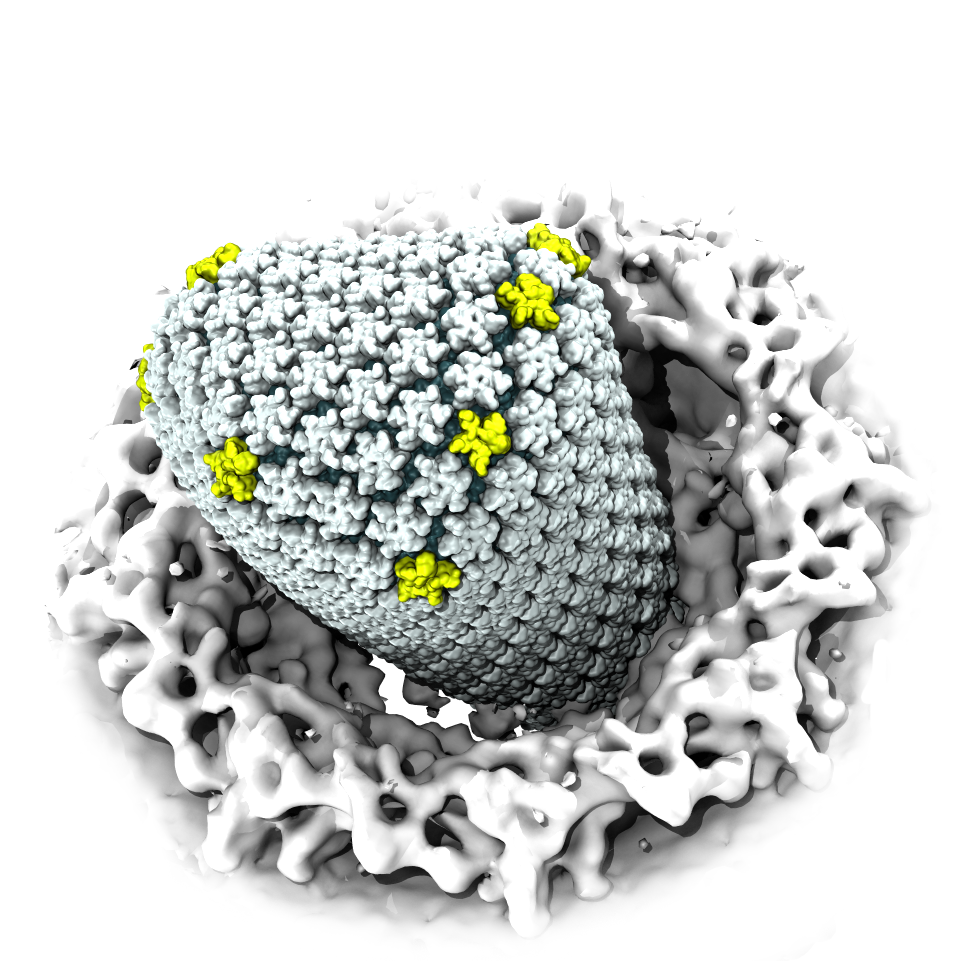
image size:
283.9KB
made with VMD
When human immundeficiency virus (HIV) infects a human cell, it
releases into the interior of the cell its capsid (made of about 1,300
identical so-called CA proteins), a closed, stable container that
protects the viral genetic material (see also June 2013 highlight Elusive
HIV-1 Capsid and August 2015 highlight Anatomy
of a Dormant Killer). Once in the cell ― while avoiding
detection by cellular proteins ― the capsid deceives the cell and
directs the cell machinery to transport it to the nucleus. The
human-cell protein Cyclophilin A (CypA) is thereby exploited to act
against the cell's well being and to assist the HIV infection by
getting the capsid to access the cell nucleus; this results in a
delicate choreography accomplished by escaping anti-viral proteins in
the cell and deceiving transport proteins at the nucleus, all of which
contain a CypA domain that interacts directly with the capsid. Despite
the availability of the crystal structure of the complex of CypA and
CA proteins determined nearly 20 years ago, the mechanism by which
CypA assists the capsid has been unclear due to the lack of
information on CypA in complex with not one CA protein, but the entire
capsid. In collaboration with experimental groups,
computational biologist have shown in a recent report that
the effects of CypA on the capsid are not only structural, but also
dynamical. Thus, new therapeutic strategies may be envisioned through
modulation of the dynamics of the capsid by small-molecule (drug) compounds
that inhibit the binding of CypA to the capsid. More information is
available on our retrovirus website and in
a YouTube video.



