Highlights of our Work
2024 | 2023 | 2022 | 2021 | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 | 2014 | 2013 | 2012 | 2011 | 2010 | 2009 | 2008 | 2007 | 2006 | 2005 | 2004 | 2003 | 2002 | 2001
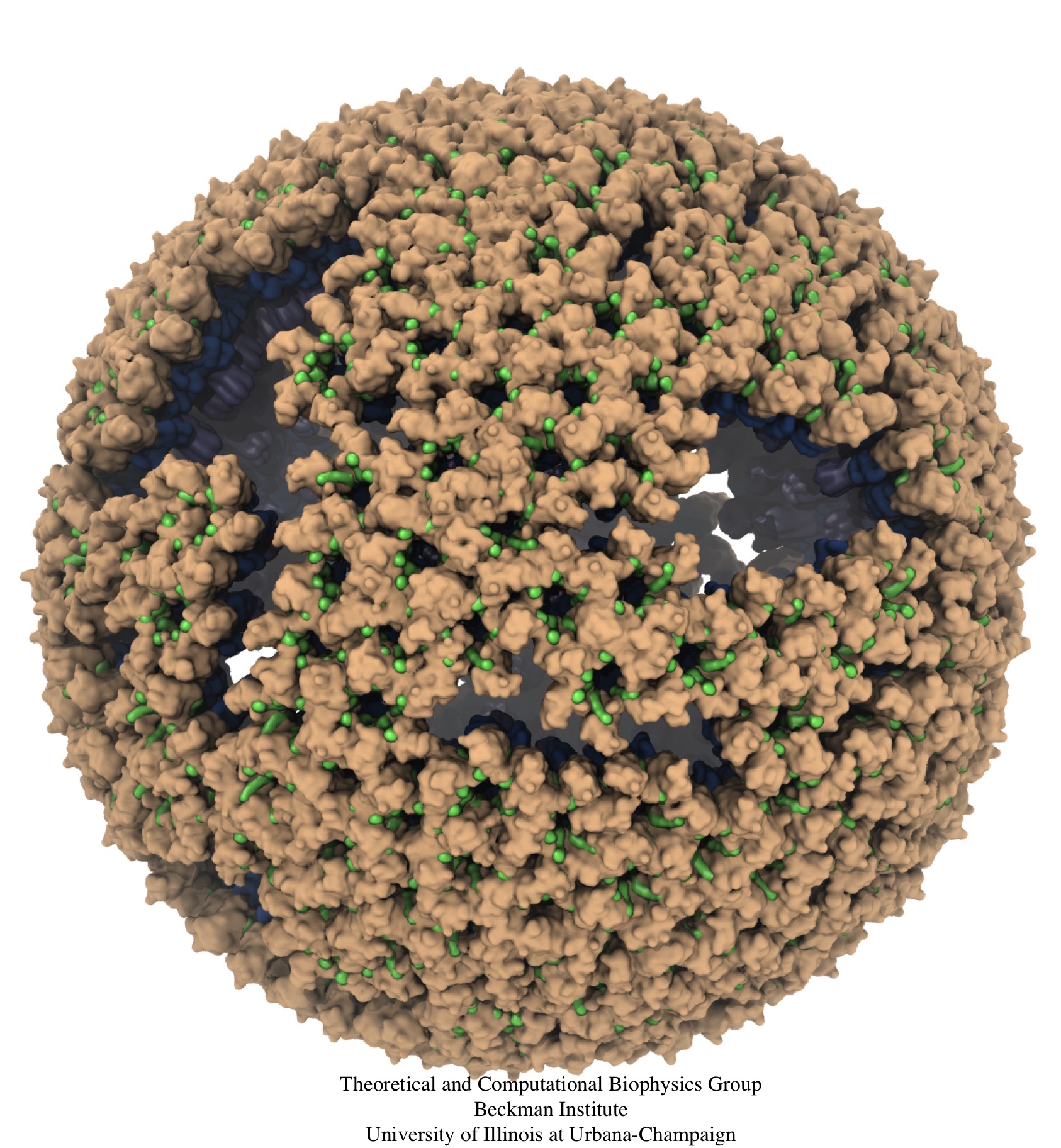
image size:
2.6MB
made with VMD
Retroviruses are parasites that pose a major health threat to humans
(for example in case of HIV) and other animals (for example in case of
RSV, M-PMV, MLV, and many more viruses).
After a retrovirus hijacks a cell, the infected cell produces
multiple copies of the virus which are then released into
the host's bloodstream.
These newly released viruses must mature before they can
infect other cells. A strategy for preventing virus spread is therefore,
to lock the viral particles in their immature, non-infectious state.
However, to render the immature virus an attractive target for
structure-based drug development one needs to know its chemical structure.
Unfortunately, the complexity and size of the viral particle ―
an incomplete hexagonal shell with a size close to 100 nm ―
have prevented the experimental determination of the chemical, namely atomic level,
structure of the virus.
As reported recently,
a team of computational and experimental researchers have provided an atomic
structure of the immature retroviral lattice for the Rous Sarcoma
Virus. The multi-domain RSV model was derived through a combination of
state-of-the-art modeling techniques, including, cryo-EM-guided
homology modeling, large-scale molecular dynamics simulations
using enhanced sampling capabilities available in NAMD,
together with experimental measurements such as X-ray
crystallography and a wealth of biochemical data.
Particularly, the model reveals novel
features of the packing and dynamics of the immature capsid protein
with implications for the maturation process and confirms the
stabilizing roles of the so-called upstream and downstream domains
of the immature RSV. More information is available on
our retrovirus website, and in
a highlight video.



