Highlights of our Work
2025 | 2024 | 2023 | 2022 | 2021 | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 | 2014 | 2013 | 2012 | 2011 | 2010 | 2009 | 2008 | 2007 | 2006 | 2005 | 2004 | 2003 | 2002 | 2001
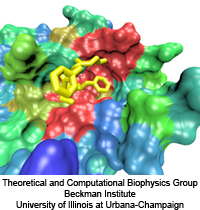
image sizes:
419.0KB
&
83.2KB
made with VMD
The nucleus is responsible for storing the genome of eukaryotic cells,
isolating it from the cellular cytoplasm. Partitioning the genetic
material is very important in protecting it from cellular processes or
foreign molecules. However, the nucleus also needs to provide access
for the rest of the cell to the information stored in the genome. Numerous
nuclear pores in the nuclear envelope offer communication pathways between
the nucleoplasm and cytoplasm. The pathways are restricted to so-called
transport receptors, proteins that taxi molecules into and out of the
nucleus. If a molecule wishes to enter or leave the nucleus, it associates
with a transport receptor. The complex passes through the pore and then
dissociates. The question is why transport receptors can pass the nuclear
pores while other proteins cannot. The answer lies in the role of
FG-repeat proteins lining the pores and filling much of their free volume.
These proteins are disordered peptides, consisting of repeating
phenylalanine-glycine (FG) residues separated by a sequence of hydrophilic
linker residues. Only proteins that interact favorably with the FG-repeat
regions can pass through, while other proteins are excluded. A
recent
report used molecular dynamics via NAMD
to examine the way in which the transport factor NTF2 interacts with the
FG-repeats. The study described binding spots for FG-repeat peptides on
the surface of NTF2, confirming known binding spots discovered previously
via experimental means, and suggesting the existence of further binding
spots. The new binding spots may play a role in steering NTF2, upon import
or export, along a particular path through the nuclear pore. See also a
previous highlight from
January 2006, "Gateway to the Nucleus", as well as our
webpage on the nuclear pore complex.



