Quantum Biology
| Fundamental biological processes that involve the conversion of energy into forms that are usable for chemical transformations are quantum mechanical in its nature. These processes involve chemical reactions themselves, light absorption, formation of excited electronic states, transfer of excitation energy, transfer of electrons and protons, etc. Some other biological processes, e.g. orientation of birds in the magnetic field of Earth, have been also suggested to require quantum mechanics. |
|
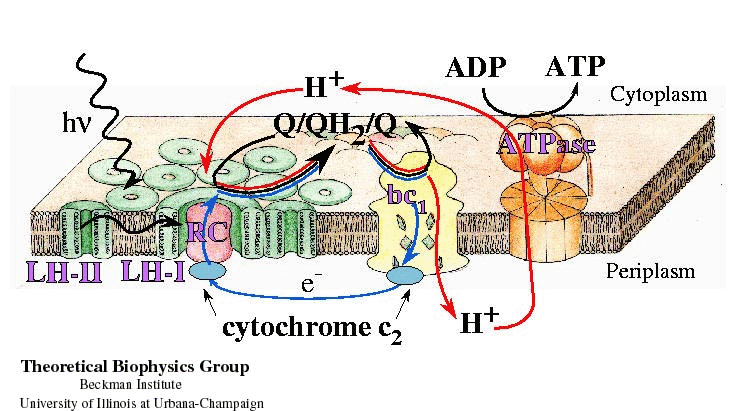
Summary of Quantum Processes required for ATP synthesis The figure presents the scheme of the integral membrane proteins forming the photosynthetic unit. Light-absorption and excitation transfer within the light-harvesting proteins (LH-II and LH-I) are represented by wavy lines. Electron transfer within the photosynthetic reaction center (RC), cytochrome c2, and bc1 complex is represented by blue lines. Proton transfer in the bc1 complex and the ATPase is represented by red lines. The chemical reaction of ATP synthesis is represented by a black line. |
| The description of quantum processes in biological systems is usually focused on specialized molecules, e.g., chlorophylls, which are embedded in larger protein environment. The extremely challenging task of describing the dynamics of such systems, involves several open problems on which the Theoretical Biophysics Group is working on: quantum mechanical description of large molecules (computational demand for quantum description increases exponentially with the number of particles), combined quantum/classical description (to treat molecules which behave quantum-mechanically within the context of its classical/protein environment), stochastic quantum mechanics combined with molecular dynamics. |
Organization of energy transfer networks in photosynthesis
|
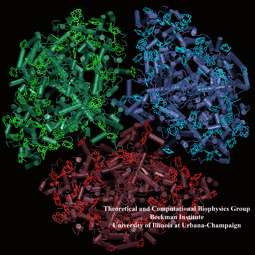
Light harvesting complexes provide fascinating challenges to biophysicists.
With the availability of atomic structures for protein-pigment complexes
such as photosystem I, it is possible to form a comprehensive picture of the
light absorption and excitation migration processes based on an atomic level
quantum mechanical description. This kind of structural analysis not only
forms a rigorous test for our understanding of the physics of these
mechanisms through a comparison to spectroscopy and kinetics experiments,
but it also provides a framework within which the organizational principles
for multi-component pigment-protein assemblies can be investigated. Figure produced with VMD . |
|
Photosynthetic Unit of Purple Bacteria
|
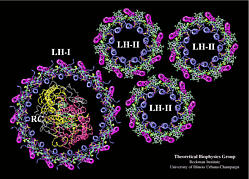
The photosynthetic unit of purple bacteria consists of the
photosynthetic reaction center, surrounded by light-harvesting
complexes. The initial step of photosynthesis, i.e., light-absorption
by chlorophylls and carotenoids in light-harvesting complexes and
transfer of excitation energy to the photosynthetic reaction center
are studied by quantum chemistry and effective Hamiltonian methods.
Figure produced with VMD . |
Light-Harvesting by Carotenoids
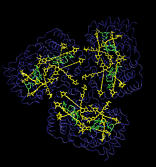
| In light-harvesting complexes, carotenoids act as
light-absorbers in the blue-green region of the spectrum. Absorption
of a photon is followed by rapid singlet excitation energy transfer to
bacteriochlorophyll (BChl). In addition to their light-harvesting
role, carotenoids photoprotect antenna complexes, i.e., they prevent
the formation of photo-oxidizing singlet oxygen by quenching BChl
triplet states through triplet excitation transfer. Light-harvesting
and photoprotection by carotenoids is studied in Theoretical
Biophysics Group in two proteins, the Light-Harvesting Complex II of
Purple Bacteria, and the Peridinin-Chlorophyll-Protein of
Dinoflagellates.
Figure produced with VMD . |
 |
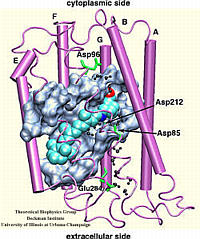
Bacteriorhodopsin
|
The all-trans retinal protonated Schiff base (RSPB) is the
chromophore of bacteriorhodopsin (bR), a transmembrane protein that acts
as a light-driven proton pump in Halobacterium salinarium,
converting light energy to a proton gradient. Upon absorption of light
the chromophore undergoes a photoisomerization process (all-trans
-> 13-cis) that eventually provides the driving force for the
translocation of protons. This elementary photoisomerization process
proceeds on multiple coupled potential energy surfaces and we have
modeled it using a formally exact quantum-mechanical procedure: the full
multiple spawning method. Currently, we are studying the first excited
electronic state of the chromophore using an isolated retinal analog
model and ab initio CASSCF methods. The characterization of the
first excited state (minima and conical intersections associated with
isomerization around different double bonds) will enable us to extend and
improve the aforementioned quantum-mechanical studies of the
photoreaction dynamics in the protein.
Figure produced with VMD . |
Magnetoreception in animals
| We study the suggestion that the geomagnetic field is detected by changes in the rates and yields of radical pair reactions. Assuming that photoreceptors involved in vision are involved in radical pair reactions, the magnetic field will result in a modulation of vision. The visual modulation patterns furnish animals with magnetic compass capabilities as illustrated in the figure. |  |
