Historical Series: Photosynthesis
Unraveling Photosynthesis Step by Step:
Four Decades of Research in Theoretical and Computational Biophysics
A History in Four Parts
By Lisa Pollack
September 2012Part 3: Intrepid Spirit Needed
Once the reaction center had been solved in the mid-1980s, its structure promised that the physics behind it could now be resolved on firm grounds. But the reaction center was not the only protein that existed in the photosynthetic apparatus of purple bacteria. Two other types of proteins were important for absorbing light: light-harvesting complex 1 (LH1) and light-harvesting complex 2 (LH2). It was known that these complexes were made of pigments that absorb photons and then transfer that absorbed energy to the reaction center. Hence the term “light harvesting” can be thought of as the process of capturing light and then transferring that energy. Structural information about LH2 was minimal in the early 1990s, which made it impossible to firmly deduce the physics behind how LH2 functioned. But all this was about to change, as two groups would race to publish their LH2 structures first.
Xiche Hu,circa 1995, who worked on solution of light-harvesting proteins.
Sometime around 1994 Hartmut Michel, now in Frankfurt, was visiting the Schulten group at Beckman, invited by Schulten to give a lecture. At a party Schulten hosted during the visit, Schulten and Michel got a bit inebriated and thus a bit emboldened, and decided to solve the structure of a light-harvesting complex Michel had been working on, even though a key piece of information was missing. Up to that point various light-harvesting proteins had been crystallized, but none explicitly solved. The two scientists made a deal: Michel's group would hand over their diffraction data, and Schulten's group would have a certain amount of time to solve the protein structure with Michel's diffraction data.
When Hartmut Michel's group began work on crystallizing LH2, another researcher at the University of Glasgow began similar determination of the structure of LH2 as well, but for a different bacterium. The British scientist, Richard Cogdell, worked on structure determination for the species Rhodopseudomonas acidophila. On the other hand, Hartmut Michel had chosen to work on LH2 of the bacterium Rhodospirillum molischianum, a decision that would have profound consequences.
Since this was such a tricky problem to solve, Schulten put a big group of people on it: three graduate students and a new post-doc, chemist Xiche Hu. Over the course of several months, as the intricacy and complexity of the problem grew and grew, everyone would drop out of the project except Xiche Hu, the others daunted by what was known as the phase problem.
When diffraction data are generated from a crystal, only two pieces of data can be extracted from the experiment. The third piece of data, missing but necessary for elucidating the structure, is the phase angles of the reflections. One way to solve this is to grow the crystal with heavy metals that are bound to particular places in the protein and through geometrical constructions determine the phase. But the protein Rs. molischianum was so modular that the experimentalists in Michel's group could not co-crystallize with heavy metals successfully. So it fell to Xiche Hu and Klaus Schulten alone to come up with a way to conquer the phase problem.
For Schulten and Hu, two blows would soon befall them as Hu worked diligently on the phase problem. First, they learned that Richard Cogdell and his collaborators succeeded in solving the structure of Rps. acidophila, and would soon publish in Nature. The light-harvesting protein that Hu and Schulten were working on turned out to be the more difficult one to solve. And second, since the allotted months had passed and Hu did not have a structure, the problem reverted back to Hartmut Michel's group. Although this relinquishment was a blow to the scientists in Urbana, Xiche Hu was philosophical about it. “In some sense they had the right to do it,” Hu remarks, “because in principle they didn't need us to solve the structure.”
Although Xiche Hu had to teach himself the entire field of crystallography to work on the light-harvesting complex, he had learned quite a few things during his limited time working with the diffraction data. So when Hartmut Michel was unable to solve the LH2 structure, he approached Schulten to rejoin the project. Xiche Hu was ready to revisit the challenge. “Xiche Hu came every morning to my office,” recalls Schulten, “and put his arm around my shoulder and said, 'Klaus, we are going to make it.' So he, not the postdoctoral advisor, was the confidence generator.”
To conquer the problem, Hu and Schulten first generated a structure that they hoped was similar to the real structure, which they called a “search model,” and which they based on the Rps. acidophila structure that had already been determined by Cogdell's group. The experimentalists in Michel's group were experts in reading diffraction data, and surmised that the structure of the light-harvesting complex was a ring made of eight identical subunits, which effectively reduced the problem to one-eighth of its original complexity. So Hu and Schulten only needed to solve the structure for one subunit in the protein, and they could utilize Cogdell's structure as a guide. In essence Hu and Schulten used a computational step to generate search models that were homologous to the unknown structure so they could determine the phases. Schulten likens the procedure to iterating with Newton's Method: if the answer diverges, one can clearly see the initial guess was far off and so one starts over with a different guess. Hu says that what finally solved the structure was exploiting the symmetry aspects of the protein. The structure they computed is shown at left.
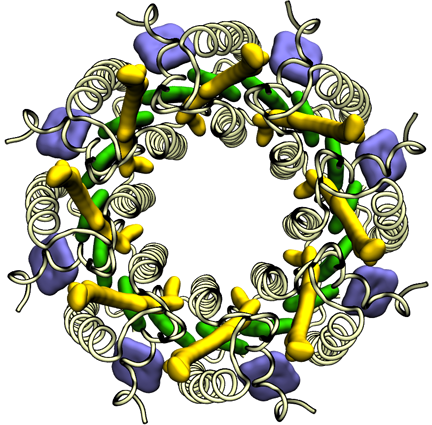
The light-harvesting complex solved by Klaus Schulten and Xiche Hu in 1995.
Schulten calls the moment when he saw the structure of the light-harvesting protein one of the peak highlights of his entire career. He realized the significance of this work immediately. His reaction, however, was not quite the same as his competitor, Richard Cogdell's. “So Cogdell tells me, every time I see him: Oh Klaus, I'll never forget when I saw the structure at first, I was just weeping,” recounts Schulten. Although the two teams were racing to solve their respective structure first, the team headed by Michel and Schulten published about a year later than Cogdell. But this publication, in May of 1996 in the journal Structure, remains one of Schulten's most highly cited papers, with 821 citations as of July 2012.
Confrontation in Spain
Although the project culminated in a good outcome, Schulten confesses he had his doubts all along the way: was the ring really eight-sided, did they have the correct symmetry group, would a useful search model ever surface? And these doubts were only exacerbated by a confrontation Schulten had at a conference, before the structure of their light-harvesting protein was successfully solved.Around 1995 Schulten went to Spain for a meeting, and gave a talk about the computational methodology Xiche Hu and Schulten were using to approach the problem of solving the structure. Basically Schulten was talking about the role the theorist could play in X-ray crystallography, a field usually reserved for experimentalists. In the audience was crystallographer Robert Huber, who had won the Nobel Prize in 1988 for his role in determining the structure of the reaction center protein. “Huber shot up afterwards, after my lecture,” recounts Schulten, “and said this is the greatest rubbish he ever heard, totally undoable and really stupid.”
Schulten thought this was way too aggressive, and he was not the only one. “There was a coffee break,” continues Schulten, “and after the coffee break, several colleagues stood up and they said: We have to comment on this, because the exchange you heard is making a very bad example for our young people.” These scientists basically conveyed that to not try new approaches, like the one Schulten was advocating, is exactly against the fabric of science. They were not dissuaded by the fact that Huber was a Nobel laureate.
Huber was privy to the whole exchange that followed the coffee break. He eventually stood up and apologized. “He said he would only be too happy if theory would replace experiment,” Schulten recalls. But Schulten recognized that Huber did not actually mean it. This whole incident was only one of many factors that contributed to Schulten's doubts about the success of the project to determine the light-harvesting protein's structure. In the end, however, his doubts were put to rest. And the structure determination was only the beginning of one of the most impressive projects ever achieved in his group.
From Structural Biology to Quantum Physics
With the structure of a light-harvesting complex under his belt, Xiche Hu set out to use the same technique he had honed in his LH2 work to predict the structure of a light-harvesting complex 1 for a different purple bacterium. Around this time two European graduate students joined the group at Beckman and started to elucidate the physics behind light harvesting, work made possible by the newly unveiled structures of LH1 and LH2. The work at Beckman would shift from structural biology to quantum biology.While he was studying for his diploma at the University of Frankfurt in Germany in the early 1990s, Thorsten Ritz heard Klaus Schulten give a seminar there about a possible mechanism for the magnetic compass in birds. Captivated by the talk, since it pointed out one of the ways in which quantum mechanics plays a role in biology, Ritz made sure to talk to Schulten while he was briefly doing research at the Center for Complex Systems Research in Urbana. Ritz decided to do dissertation research with Schulten in Urbana while getting his PhD from the University of Ulm. As Schulten did not have a candidate molecule yet for the avian magnetic compass, Ritz began his studies on photosynthesis instead.
|
"THE EXCHANGE YOU HEARD IS MAKING A VERY BAD EXAMPLE FOR OUR YOUNG PEOPLE." Klaus Schulten |
In a similar vein, Ana Damjanović was an undergraduate student in physics at Belgrade University when she met Schulten at a conference in Europe. Impressed by his research, she went to the University of Illinois at Urbana-Champaign specifically to get her PhD in Schulten's group. Shortly after her arrival, she would team up with Ritz and begin work on the quantum physics of photosynthesis.
One of the first things the trio would immediately uncover was how quantum coherence assists light harvesting. In the two light-harvesting proteins, LH1 and LH2, chlorophyll-like molecules (technically called bacteriochlorophylls in purple bacteria) are packed closely together in a ring shape, as seen in the picture of LH2 for example. To transfer the energy harvested by the chlorophylls, the individual chlorophylls team up and transfer the excitation not randomly but in a pool. They share their excitation in a very ordered, or so to speak, “coherent” way; it is as though they are humming one tune together as opposed to each playing unique parts in an orchestra. With quantum coherence, the system of pigments could reach very far and fast to transfer the excitation.
In the 1940s Arnold and Oppenheimer had wondered why so many chlorophylls were necessary in photosynthesis and exactly how they all worked together. In the late 1990s, Schulten together with Hu, Ritz and Damjanović elucidated the physics underlying the structures of the light-harvesting proteins, namely that the arrangement of a group of tightly-interacting chlorophylls in fact act together to make themselves more efficient through quantum coherence.
Return to Polyenes
The light-harvesting pigments are not only made of chlorophyll-like molecules that absorb sunlight. They also consist of carotenoids, which are pigments that also absorb light, usually in the blue range, and are popularly known for their nutritional value in foods such as tomatoes and sweet potatoes. The other main highlight of the collaboration between Damjanović and Ritz was clarification of the role that carotenoids play in light harvesting. Basically they found that the carotenoids have to use tricks in order to transfer the excitation to the chlorophylls.
In this study of carotenoids, a trip to Japan for an unrelated reason rekindled Schulten's interest in his Harvard work from the early 1970s on polyenes. Carotenoids are related to polyenes, as both share an underlying structure of conjugated double bonds, and both polyenes and carotenoids have low-lying, optically forbidden states. While Schulten was in Japan in the late 1990s for a conference on visual receptors, he met Yasushi Koyama, an experimentalist who had studied the forbidden states in polyenes. Koyama was keen to collaborate with Schulten and, in order to get to know him better, asked Schulten if he would like to take a drive to an earthquake museum. The Kobe earthquake rocked Japan in 1995, and a memorial museum commemorated the devastation.
During the trip to the earthquake museum, Koyama revealed he was aware of the work Schulten and Karplus did in the early 1970s to prove that polyenes had optically forbidden states, and that he was very eager to team up with Schulten's group for a paper. Schulten and Koyama, together with the team of Ritz and Damjanović, elaborated on the mechanism that made carotenoids sometimes couple efficiently to chlorophylls to transfer excitation when the coupling was expected to be poor. They suggested in a 2000 paper that some photosynthetic systems have a type of symmetry breaking in their carotenoids that augments the excitation transfer efficiency.

Klaus Schulten, Ana Damjanović and Thorsten Ritz, who elucidated the quantum physics of photosynthesis, circa 1998 or 1999.
This period of time in Schulten's group, from about 1995 to 2000, still stands out as one of the most impressive eras of achievement in Schulten's mind. It started with Xiche Hu and his relentless efforts to solve the structure of LH2, and then ended with Thorsten Ritz and Ana Damjanović. The pair basically sorted out the key physical characteristics that nature had designed to effectively absorb sunlight, to keep it for a short while and transfer it effectively to other pigment subsystems in the so-called photosynthetic light harvesting system.
When asked why the team of Hu, Ritz, and Damjanović was able to unravel the physics behind light-harvesting in such a compact amount of time, Damjanović points to three reasons: the team worked extremely hard, she and Thorsten Ritz helped each other cooperatively, and when stuck, Damjanović took full advantage of the many resources available. “There was usually someone around who knew how to use the software or programs that I needed to use,” Damjanović clarifies, “If I got stuck somewhere, I would go and ask them for help.” This way Damjanović avoided gridlock in the research, by promptly seeking help from scientists at the University of Illinois, and sometimes even researchers at other institutions.
Schulten also weighed in on why the team of Ritz and Damjanović made remarkable achievements. Xiche Hu's work on the light-harvesting protein was the first time in Schulten's group that someone had worked on structural biology. “This structure determination was very new for us; but particularly relative to the structural biology, we were really excellent quantum biologists,” notes Schulten. This second hat that the team wore, of quantum physicists, was a natural one for Schulten's group to embrace since they could draw on years of experience, and this resulted in explanation of the quantum physics behind light harvesting in photosynthesis in the near-record time of about three years.



