Historical Series: Photosynthesis
Unraveling Photosynthesis Step by Step:
Four Decades of Research in Theoretical and Computational Biophysics
A History in Four Parts
By Lisa Pollack
September 2012Part 1: Early Influences on Klaus Schulten
“Today you think it's exciting, but in those days it was considered the most boring thing you could do, to study molecules,” remarks Klaus Schulten on his decision in 1969 to pursue a PhD in molecular physics. “That was done in the 1930s, and even then it was boring.” But Schulten had a master plan, and he surmised molecular physics was the right field to pursue in graduate school, never tempted by the discoveries promised in the hot new field of particle physics. He wanted to study biology, but specifically at the level of atoms and molecules, and he imagined that physics would give him the tools he needed to accomplish this.
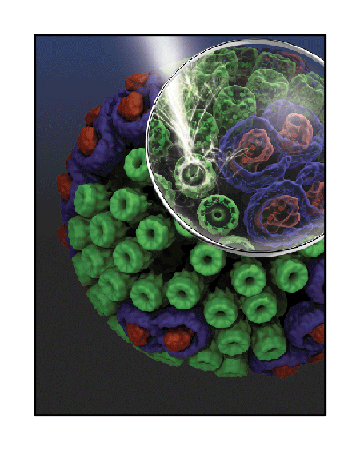
The chromatophore, a compact cellular compartment, as envisioned by Klaus Schulten's research group.
From a very early age Schulten was fascinated by biology. A voracious reader in all areas of science, he discovered, around age twelve, Du und das Leben (You and Life), a book by the eminent biologist Karl von Frisch, and the contents captivated him. “He was a great biologist who very early on appreciated the molecular basis of living systems. So he brought the two together and wrote on what life is, but that it comes from the molecules.” In fact, there was one particular chapter that caught his attention, about how cells employ photosynthesis to make ATP, the molecule that fuels life. “I felt that was my calling,” Schulten says; “I wanted to understand how life forms use energy to make ATP and then run many processes with it.”
Through high school Schulten continued to read on his own about science, finding very little stimulating on it in the classroom. In fact, because he liked to challenge his teachers with the things he taught himself, he was given the nickname “Professor” by his classmates. “When I got my degree at the high school, the teachers asked me, what do you want to study, and I said I want to study it all: chemistry, physics, mathematics, and biology. And that, of course, was further proof for them that I was totally crazy.”
When Schulten was in his early twenties, studying for his diploma at the University of Münster, he often walked in its famed botanical garden with fellow students. “I discussed at length with my friends what I wanted to do with my life, and that is exactly what I do today.” But what he wanted to do didn't exist at the time–he wanted to look inside living cells, at the level of electrons and atoms, to understand how they functioned; and he wanted to do it theoretically instead of experimentally.
The story charted in the following pages will reveal how a life's calling was realized over the course of forty-plus years. In its beginning stages, a tapestry of people in Schulten's early adulthood, when woven together, led to a familiarity and then a fascination with photosynthesis. Despite this fascination, Schulten at one point had to wait ten years before he could do the kinds of calculations he deemed worthy of publication: “What I really like about the photosynthesis is it goes from the molecule, even the electron, to the whole cell. And that is basically what I always wanted to do; that is why I stuck with it for a long time.”
Instead of plants, Schulten chose to study purple bacteria, which embody the simplest form of photosynthesis. Bacteria emerged around 3.5 billion years ago on the earth; photosynthesis, an ancient process, arose sometime thereafter, although no firm evidence exists to prove its exact origins. While many associate it with plants, photosynthesis most likely originated in bacteria, and photosynthesis is found in many different phyla of bacteria today, purple bacteria being one of them. The purple bacteria are a gift for scientists studying photosynthesis, as the machine or unit that runs photosynthesis in these bacteria is simpler than the ones in plants.
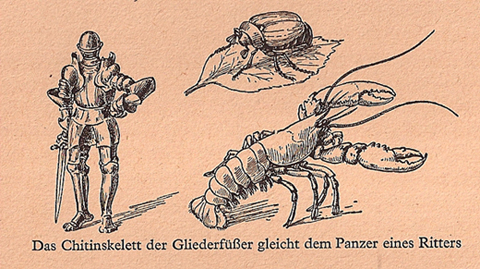
Image from Du und das Leben, a book that captivated the young Klaus Schulten.
Even in its least complicated incarnation, Schulten never dreamt that he could understand thoroughly this phenomenon when he started the field in the 1970s. But forty years of dedication has paid off. “At the end, today,” summarizes Schulten, “I think we are pretty close to really going full circle and understanding everything.” This history documents how the pieces all fell together and, after setbacks and dry-spells, resulted in an impressive compendium of work by many scientists from different fields who worked with Schulten on a topic so key for life on earth.
Graduate School at Harvard: The Invisible States
When Schulten finished his diploma in physics at Münster, he carefully considered his next step, one that would bring him closer to his childhood dream. As a youth he had a mathematical mind and a love for living systems. At the school aquarium he wanted to understand the fish and the plants, and why they were alive. “I was never interested in just a feather, or in a bone,” he recounts about his youth. “It was more like this living entity that all comes together, that's what interested me.” When deciding on graduate schools, he asked around and the only person at the time who combined physics with living systems was Martin Karplus at Harvard.
Formally trained as a theoretical chemist, having worked with Linus Pauling at Cal Tech in graduate school, Martin Karplus spent many years doing chemistry-related research before he returned to his first love, biology. In the fall of 1969 he took a leave of absence from his position as a chemistry professor at Harvard to visit the Weizmann Institute in Israel to uncover how the tools of a theoretical chemist could be applied to biological problems. He came back from the institute with a host of problems in biology to study, his stay a success.
Around this same time, Klaus Schulten decided he wanted to work with Karplus on his PhD, for he knew Karplus combined mathematics, physics, chemistry and biology in his research, a long-held goal for Schulten. With a fellowship from the Volkswagen Foundation, Schulten began his studies, and would eventually receive a degree in chemical physics.
One of the areas of study Karplus chose to pursue after his stay in Israel was the role of retinal in vision. Retinal is related to the carotenoids, for basically half a carotenoid molecule is vitamin A, and when oxidized vitamin A becomes retinal. Retinal also is one example of a polyene, a type of chemical compound with multiple carbon-carbon bonds. Polyenes play key roles in light absorption in vision as well as in photosynthesis. Studying polyenes would become a major focus of the Karplus group at Harvard.

Martin and Marci Karplus, circa 1976.
Photo used with permission.
Karplus, in his various jobs over the years, always appreciated the fresh perspectives he gained from talking to the other colleagues at his institution. One of the fuels that fed Karplus's polyene research came from interactions with a new assistant professor in chemistry, Bryan Kohler, whose experiments intrigued Karplus. With his graduate student Bruce Hudson, Kohler had found that when they shined light on certain polyenes and looked at the resulting excitation bands, there was a little excitation they couldn't explain. The wisdom at the time held that there was some dirt in the solution they couldn't clean away, which was responsible for this weak absorption. “So then they cleaned and cleaned but it didn't go away,” recalls Schulten about this mystery. “And they went to my advisor, Karplus, and asked him if there is a chance that there is some electronic excitation there. I was just a new student of Karplus's, and so when you have a new student you also have a new victim.”
Karplus and Schulten decided to employ the so-called electron correlation effect to see what would happen if they excited a pair of electrons in the polyene in a coordinated manner. “You might say if you lift two electrons up that's crazy,” says Schulten about this theoretical approach. “That costs twice as much energy, but that's not necessarily so. And because the electrons might do it in such a coordinated way that the excitation is actually lower in energy than moving just a single one, that is what I was going to try out.”
Schulten started this work in the fall of 1971, and actually stayed in Boston over the subsequent winter break to investigate this new problem and prove the existence of forbidden, low-lying states. He vividly remembers a snowstorm during that time, which almost paralyzed the region; Schulten was barely able to get out and buy food. But that was not his greatest concern: “I was always worried that Kohler and Hudson would come through the door any moment and tell me, 'Oh, sorry, we found a better cleaning agent and now the effect is gone.'”
Because it was winter break, Schulten had the office and computer center all to himself. “I worked there day and night,” Schulten recalls. “Basically I remember that I only stopped working when my hand got cramped from holding the pen, because I had to derive all these quantum mechanical rules that describe the system.”
Schulten was very careful, however, and even did the calculations two or three times to make sure he made no mistakes. His diligence paid off; when Karplus returned from break, Schulten showed him proof of the mysterious low-lying states. “These other states were not seen because they are what physicists would call magnetic,” explains Schulten. “They actually involve the spins, and spins are basically magnetic moments.” His work revealed that polyenes have certain triplet-triplet excitations responsible for the feeble excitations the experimentalists witnessed. (A triplet has two electrons whose total spin adds to unity.) “If you take two triplet excitations in the polyene and add them up,” spells out Schulten, “you are getting an excitation energy that is still lower than the lowest excitation that is seen in an optical transition.”
|
"WHEN YOU HAVE A NEW STUDENT YOU ALSO HAVE A NEW VICTIM." Klaus Schulten |
Publishing this new finding, however, was not straightforward. Other researchers claimed that what Kohler and Hudson saw was a well-known “dirt effect” and not something real. They actually claimed the dirt was in the window of the cuvette and not in the polyenes themselves. Schulten, a rookie in computational biology at that point, summarizes how the paper was likely received by some in the scientific community: “Do you know what foolish thing Karplus did? He wrote a paper on this dirt effect.” But Schulten and Karplus persevered and eventually published their findings. Schulten's understanding of these polyene excitations would later inform his photosynthesis research.
While Schulten was applying techniques of theoretical chemistry and physics to biological problems in his formal studies, his life outside the classroom at Harvard was enriching his mind just as much, and helping to shape his future research interests. He loved to spend free time in the biology labs of George Wald and Ruth Hubbard, a power couple on campus. “Several times they threatened to kick me out,” recalls Schulten, “because I was too arrogant. I was a physicist, and so I always thought I was so much smarter than all the biologists.” George Wald received the Nobel Prize in 1967 for having discovered that retinal is used by all species with eyesight. Vision, like photosynthesis, is a process involving the interaction of biomolecules with light and Schulten learned about retinal and the carotenoids from Wald. “He was a real big influence on me,” Schulten muses; “I was interested in vision but then I knew of course that these kind of molecules also play a role in photosynthesis.”
The Boston area around Harvard was a vibrant environment, especially for young scientists. While living there Schulten also heard about some work on carotenoids by the Tufts biochemist Norman Krinsky. Known later as the father of modern carotenoid research, one of the many things Krinsky worked on was studying the protective role of carotenoids. Although carotenoids are pigments that absorb light, Schulten heard about research that proposed another role for the carotenoids: as quenchers of excited oxygen. Krinsky and many others working in the field in the 1960s and 1970s found that the length of carotenoids was related to their protective function, and that mutant strains with shorter-than-normal lengths would not exhibit protection for the bacteria; that is, bacteria with shorter carotenoids would die when exposed to light. Schulten grew fascinated upon hearing about this other role for carotenoids, as protectors of living organisms.
Max Planck Institute for Biophysical Chemistry
After finishing his PhD, Schulten returned to Germany to work at the Max Planck Institute for Biophysical Chemistry in Göttingen. Dozens of different Max Planck Institutes exist all over Germany, conducting innovative research in many fields of science and the humanities. Albert Weller, an institute director in Göttingen who hired Schulten, ran an experimental group focused on characterizing electron transfer reactions. Weller immediately gave Schulten the job of working out an oddity in one of Weller's pet reactions, namely why something called “fast triplets” were observed. In this reaction the scientists saw a mysterious product that was an excited molecule with a pair of electrons whose spins were parallel, hence a triplet. The product occurred faster than normally expected.Schulten got to work straightaway. “I figured out where it comes from,” he explains. “And I also figured out that you could verify it by doing the reaction with and without magnetic fields.” What Schulten essentially discovered is that a magnetic field can influence a chemical reaction, a new physical effect. He immediately published an initial, qualitative paper suggesting the effect, and then, teaming up with his wife, Zan Luthey-Schulten, and Hans-Joachim Werner, a student in the group that Schulten was mentoring, they presented the full quantitative theory of the effect in two papers published in 1977.

Albert Weller, a director of the Max Planck Institute for Biophysical Chemistry from 1971-1990. Image taken from here.
Because the computing facilities at Göttingen were not stellar, the three had to use sophisticated mathematical tricks for this mammoth feat. “We achieved a numerical solution,” recounts Schulten, “of the problem of how two molecules that transfer electrons between them, and carry their unpaired electron spins that move around and back, how they can react with each other in a solvent.”
Around 2010 Schulten found himself refereeing a paper for Nature by a well-known physics group, and was surprised to discover that they presented exactly the same computations that the three theorists at the Max Planck Institute had painstakingly carried out over three decades earlier, without the benefit of sophisticated computers. As Schulten sees it, “something that people were proud of in 2010 we did in 1976.” But Schulten and his collaborators were not satisfied with just detailing the qualitative theory behind this new magnetic field effect. They began to think about all the ways it could be applied.
In electron transfer, an electron moves from one place to another, a seemingly simple process. But Weller and collaborators had worked for many years fine-tuning a system of donor and acceptor molecules that was optimal for making measurements in the laboratory. While Weller's pet system, pyrene and dimethylaniline, was a chemical system, the scientists who worked for Weller at the institute were fully aware that electron transfer was an important process in biological systems as well. Since Schulten knew about electron transfer in biological systems, he started thinking about application of the new magnetic field effect to biological systems, ones beyond the chemical systems favored in Weller's group. “Basically, when you have a new physical effect,” notes Schulten, “like this magnetic field effect, you very often can use it as a measuring stick.” But what kind of measuring stick is optimal? “The measuring sticks that you usually are looking for are ones that observe processes that are too miniscule in terms of size, and too fast in terms of time, to observe easily in experiment.” In other words, all one needed to do was put the magnetic field on the system, then take it away, and see if there was a different outcome.
Electron transfer processes were known to be involved in photosynthesis. Being in an electron transfer group, Schulten says that in the back of everybody's minds was an awareness of just how important electron transfer was to photosynthesis. So in 1978 Schulten and Werner, along with their director, Albert Weller, published a paper suggesting that the new effect could be used as a ruler to characterize in more detail the electron transfer that occurs in photosynthesis. The goal was to use the magnetic field effect to understand better the electron transfer in photosynthesis.
There were many reasons why Schulten already knew enough about electron transfer in photosynthesis to suggest using the magnetic field effect as a yardstick in his 1978 paper. The electron transfer in photosynthesis occurred in a protein called the photosynthetic reaction center, which may be thought of as the heart of the photosynthetic unit. When plants and bacteria absorb energy from the sun, this energy is used by the reaction center to transfer an electron. The workings of the reaction center were not fully understood because the structure of this protein was not known in the late 1970s when Schulten was in Göttingen. “So people actually did very well,” recalls Schulten, “to conclude, from certain optical properties and from electron transfer rates, what the structure looked like. So it was amazingly good actually, but it was not firm.”
One of the groups that did, however, study these electron transfer reactions, despite the uncertainty about the reaction center structure, was located in the Netherlands, in Leiden. Schulten knew them and their work quite well. Led by Arnold Hoff, they studied magnetic resonance in photosynthesis, and Schulten was impressed by how far they got without knowing the exact structure of the reaction center. When Schulten published the 1978 paper suggesting that the new magnetic field effect be used as a yardstick to characterize electron transfer in photosynthesis, Albert Weller, his boss, was one of the co-authors. Referring to this paper, Arnold Hoff told Schulten that Hoff should have been an author on the publication too. “Now Hoff didn't do anything,” recounts Schulten, “so he didn't have a reason to be an author of this paper. But he said that Weller had even less of a reason.” Schulten did not buy this argument, as he felt that Weller ran a great institute that inspired and enriched him as a scientist. “People ignore a little bit the role that the big guys play,” says Schulten. “They not only give you a seat to sit on, they also give you ideas. They sell the whole of science by saying why it is important, and what will be done in the next few years. If they wouldn't do that, one couldn't do one's work.”

Klaus Schulten, in his office in 1978 at the Max Planck Institute in Göttingen.
While Schulten knew about the Leiden group and their work, another reason he was aware of current photosynthesis research he attributes to being in Germany. “Science has strong local aspects,” he explains, “so that certain feats of science are done more in one country than another, for example. And photosynthesis was studied a lot in Germany.” One of those hubs was located in the Technical University of Berlin, at the Max-Volmer Institute for Physical Chemistry, which was really put on the map by Horst Witt. Schulten visited the institute often and learned a great deal about Witt's efforts, which focused on explaining oxygen-generating photosynthesis. “He was very bold,” Schulten notes. “He did it actually before one knew much, but he really struggled hard to give a picture of what could be involved. And that really also gave me a broader picture of photosynthesis as a biological process that involves many different steps and many different proteins. That motivated me to understand how living cells actually combine many steps to have a good outcome.”
Besides the groups in Berlin and Leiden, Göttingen also shaped Schulten's views on the complexity of biological processes that would later inform his own photosynthesis work. One of those Göttingen influences was a man who worked there at the institute, Manfred Eigen. In 1967 Eigen received the Nobel Prize in chemistry for having studied very fast reactions. Although trained in physics and chemistry, his attention turned to biological processes later in his career, especially to the origin of life. “Here we have the molecules,” Schulten says of Eigen's views on biological organization, “and here we have the whole cell: surviving, producing enough energy for its survival, and doing so many levels of reactions. And to begin to understand the challenges involved in organizing all those levels was really the program of Eigen's intellectual life in a way.” This influenced Schulten to always consider biological organization in a living cell.
While Schulten went to lectures by Eigen in Göttingen, he also participated in the famous annual gathering known as the Winter Seminar, in Klosters, Switzerland. There Schulten learned to ski during the days and listened to cutting-edge talks at night. Started in 1965, this gathering first encompassed only coworkers at the institute. In an online video, Manfred Eigen discusses how the Winter Seminar originated: “We should go away from the lab, because in the lab you have your daily life here and you should really go to some nice silent place. And since there are so many meetings in summertime, you say, let's have a winter seminar and let's discuss there our problems. And if we have time left, let's go skiing.” Later the seminar grew in popularity and attracted an international following; Eigen estimates that between 30 and 50 Nobel Prize winners attended in the course of its run over several decades. The seminar was unique, as it purposely did not require an abstract and manuscript and completely finished work, as most meetings did at the time. “In other words,” continues Eigen in a second video, “people are uninhibited to talk about things because they are not pinned down. They can speculate in the seminar, and that makes it lively.” Being near a force like Manfred Eigen, who wanted to understand biological complexity, encouraged Schulten to think of photosynthesis as an overall process that, although it involves many steps, should be viewed as a unit.
Göttingen also brought Schulten's attention to a critical equation by physical chemist Theodor Förster, via his supervisor Albert Weller. Schulten knew about the various accomplishments of German Theodor Förster because Weller had worked with Förster for many years studying protolytic reactions in the 1950s. From 1946 to 1949 Förster published three papers that described how an excited molecule transfers its excitation to an acceptor molecule. Soon becoming known as Förster Resonance Energy Transfer, or FRET, this mechanism now made it possible to describe how one chlorophyll transfers its excitation to a nearby chlorophyll during photosynthesis. “Förster made a tremendous contribution to science with it, way beyond photosynthesis,” remarks Schulten on this equation. “His formula and his idea are used today all over the world in many laboratories.”
While the accomplishments of Förster and his famous equation came to Schulten naturally through Weller's lineage to Förster, Schulten is quick to point out that Förster was not the only one in the 1940s to wonder at the mechanism of excitation transfer between pigments in photosynthesis. The famous physicist Robert Oppenheimer, known for his work on the Manhattan Project during World War II, published in the minutes of the American Physical Society a brief abstract in 1941 entitled “Internal Conversion in Photosynthesis,” right before he got caught up with the war effort. In the abstract he addresses excitation transfer from certain pigments (namely, fluorescent dyes) to chlorophylls in photosynthetic algae, suggesting the transfer is a large-scale model of internal conversion of gamma rays. After the war, in a 1950 paper with William Arnold, the pair elaborated on the mechanism of excitation transfer between chlorophyll and phycocyanin, a pigment used as a dye. William Arnold had a background in physics and biology and had worked on experimental aspects of photosynthesis since the 1930s. Arnold had done some work to show that light absorbed by the pigment phycocyanin was transferred to chlorophylls instead of directly reducing carbon dioxide. When he told Oppenheimer about this finding, they set out to explain the mechanism of excitation transfer. While the pair discussed this idea of excitation transfer as internal conversion sometime around 1939 or 1940, they did not get around to publishing this explicitly until 1950, according to Arnold's autobiographical article.
|
"GOETTINGEN WAS THE PLACE WHERE MY SCIENTIFIC DIRECTION WAS SHAPED" Klaus Schulten |
But why is the Oppenheimer and Arnold work still relatively unknown? Schulten notes that their abstract approach was not particularly helpful for biologists. “Förster was more of a physical chemist, not a physicist, and he had a much better way of explaining what he learned,” says Schulten, about the difference between Förster's and Oppenheimer's strategies. “In particular Förster described very carefully the elementary act of sharing the excitation between two chlorophylls. Oppenheimer knew that, too, but then he immediately averaged over hundreds of them. But what was really very important later was to know the pair behavior.”
Although Schulten published his first paper ever on photosynthesis in 1978 while at the Max Planck Institute, his next foray into the field would come only after a ten-year hiatus. But the serendipitous opportunity that presented itself a decade later would prove a treasure trove for a computational biophysicist like Schulten. The reason for the hiatus was because Schulten realized that for his theoretical purposes, the data was just not there–the structure of the reaction center was still unknown. “One could have done more,” says Schulten, “but it would have always been questioned.”
Living Among Experimentalists
From 1974 to 1980 Schulten worked in Göttingen, and although he only published one paper, among many, on photosynthesis, his time at the Max Planck Institute was critical to his future work in the field. “Göttingen was the place where I learned the most and where my scientific direction was shaped most,” Schulten notes. “And that was clearly due to the fact that I lived among experimentalists.”
Schulten was surrounded by 40 or 50 experimentalists who worked in his group. Schulten calls himself a mathematician by ability, who has a love for life sciences, but acknowledges that, back in the 1970s, it was tricky to apply mathematics to a new field like biology; there was much resistance and uncertainty. Schulten realized that to tackle this precarious task of introducing mathematics to biology he would need to work on solid ground and become thoroughly familiar with the experimental side. His time in Göttingen provided just that opportunity.
First, his experimentalist coworkers questioned Schulten's pure theoretical approach, which only made him improve his methods; there were also lots of good-natured jokes about him being a theoretician. Second, Schulten figured out how to apply a critical eye to experiment. “You need to know what are they doing, how are they doing it, who are the good people, and who are the not-so-good,” Schulten remarks. “So somehow you need to develop a working relationship.” Third, he learned about key directions in the field and heard about cutting-edge experiments while at the institute. In short, his time in Göttingen was not only productive in terms of research, but also because he made many contacts, with experimentalists in particular.



