Highlights of our Work
2025 | 2024 | 2023 | 2022 | 2021 | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 | 2014 | 2013 | 2012 | 2011 | 2010 | 2009 | 2008 | 2007 | 2006 | 2005 | 2004 | 2003 | 2002 | 2001
Inorganic nature brings about symmetry that one can admire,
for example when one sees a polished diamond.
Living nature, too, brings about symmetric structures;
for example, many processes in living cells are carried out
by highly symmetric protein complexes. In case of living cells
symmetry comes about partially out of physical or geometrical necessity,
but also partially out of its usefulness for the intended purpose.
Hence, understanding symmetry in biology goes beyond related studies
of the beauty of symmetry in the physical and mathematical sciences
as one also asks: Does symmetry help?
Put it another way: Symmetry in living cells is beautiful and useful!
The proteins in symmetric complexes are often imaged by electron microscopy (EM),
but unfortunately not at a resolution high enough to recognize chemical detail,
as is needed in most cases, e.g., in case of the study of a symmetric multi-enzyme system.
Computational biologists have developed a method to solve the resolution problem,
using high resolution images obtained through X-ray scattering of related molecules and
matching them through molecular dynamics using
NAMD
to the image seen in EM. This method, called molecular dynamics flexible fitting (MDFF) has been
highlighted
previously and is described on our
MDFF website.
As reported recently,
MDFF has now been extended to determine the atomic-level structure of symmetric multi-protein systems.
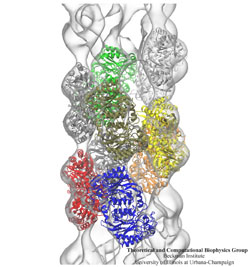
MDFF has been applied successfully to three highly symmetric multi-protein systems:
(i) GroEL-GroES, a protein complex that assists proteins to fold properly into their native conformation;
(ii) a nitrilase multi-enzyme system that converts massive amounts of molecules into forms
more suitable for a bacterial cell;
(iii) Mm-cpn, a protein complex supposedly involved in assisting protein folding
in so-called archaebacteria.
In all cases the symmetry of the protein complexes plays a key role.
For more details, see the
Method
section of our
MDFF website.