Highlights of our Work
2025 | 2024 | 2023 | 2022 | 2021 | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 | 2014 | 2013 | 2012 | 2011 | 2010 | 2009 | 2008 | 2007 | 2006 | 2005 | 2004 | 2003 | 2002 | 2001
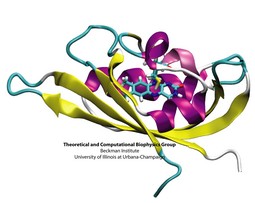
image size:
162.1KB
made with VMD
Plants and other photosynthetic organisms convert sunlight into various forms of metabolic energy. To expose themselves optimally to the sun while at the same time avoiding damaging overexposure to light, these organisms employ molecular photosensing systems that control, for example, the orientation of their leaves. Common photosensing systems include photoreceptors of the so-called phot family that are sensitive to blue light and contain Light, Oxygen, and Voltage (LOV) sensitive protein domains as photoactive elements. Light absorbed by a flavin molecule leads to bond formation with the protein (LOV domain), thereby, initiating signaling until the flavin-protein bond breaks spontaneously. A study of the photosensing events in the LOV domain of the algae C. reinhardtii (see figure), reported in a recent paper, employed computer simulations that combined quantum mechanical and classical simulation methods to study photoexcitation and subsequent processes. It emerged that formation of the flavin-protein bond is initiated by a unique light-driven transfer of a hydrogen atom between the LOV domain and the flavin molecule (see our biological photoreceptors website).



