Nanoengineering
Computational Modeling Assists Engineers in Developing Nanopore Devices for
Sequencing DNA
Our group, the Theoretical and Computational Biophysics Group (TCBG) at the
UIUC Beckman Institute is involved in a collaboration to develop a new device
for DNA sequencing. The device is to be built around a 2-nm-diameter pore in a
thin (2-5nm) silicon membrane. The chemical sequence of a DNA molecule can be
discerned by such a device in principle through semiconductor detectors,
integrated with the pore, that record electrical signals induced by the DNA
molecule passing through the pore. In order to relate the DNA sequence to the
measured electrical signals it is essential to characterize DNA conformations
inside the pore in atomic detail. Indeed the DNA translocation process can be
investigated at atomic resolution through Molecular Dynamics simulations. To
assist electrical engineers in developing such nanopore sensors, our group is
conducting molecular dynamics simulations of DNA translocation through synthetic
nanopores in an ongoing collaboration with UIUC electrical engineers. The work
has recently been reported in two publications (paper1, paper2).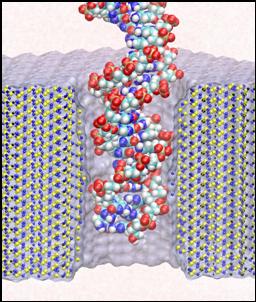
Molecular dynamics simulations provide dynamic images of nanodevices as a kind of computational microscope, that images as well as analyzes nanometric devices. In this method, a molecular system is approximated by an ensemble of virtual atoms interacting with each other according to the molecular force field, which has been developed and calibrated to reproduce quantitatively physical properties of the simulated system. A typical system simulated by TCBG members includes a patch of a silicon membrane dividing electrolyte solution into two compartments connected by the nanopore. A DNA molecule is placed in front of the pore. External electrical fields induce capturing of the DNA molecules by the pore from the solution and subsequent translocation. Measuring duration of DNA translocation at experimental fields brought about estimates of the resolution that the bio-electrical sensor should have in order to detect DNA sequences. Visualizing interaction of DNA with the surface of the pore revealed hydrophobic adhesion of DNA bases to the pore walls that may considerably slow down or halt DNA translocation or even induce unzipping of double strands. Varying geometry of the pore in MD simulations allows screening of possible DNA conformations and identifying those that produce strongest sequence-specific electrical signals. Future work will address design of ideal nanopore shapes, coatings of nanopore walls, application of mechanical forces on one or both sides of the nanopore to translocate DNA in a more controlled way, as well as the application of various types of electrical field, e.g., alternating fields. The future simulations will also study the signatures in various electrical recordings that can be obtained in principle, along with the error bars expected.



