Overall efficiency of a photosynthetic vesicle
Efficient Energy Harvesting in Purple Bacteria
Most life on Earth is powered directly or indirectly by harvesting the energy of sunlight. Plants and bacteria convert solar energy into chemical energy, which is used, ultimately, for producing food. Compared to the complicated energy harvesting apparatus of plants, the primitive purple bacteria display a far simpler instance of photosynthesis. In purple bacteria, the energy harvesting processes are performed by a spherical vesicle of approximately 60 nanometer diameter called the chromatophore. The structure of the chromatophore, including proteins essential to energy conversion such as the cytochrome bc1 complex and ATP synthase, has been determined by a collaboration involving experimental and computational methods (article).
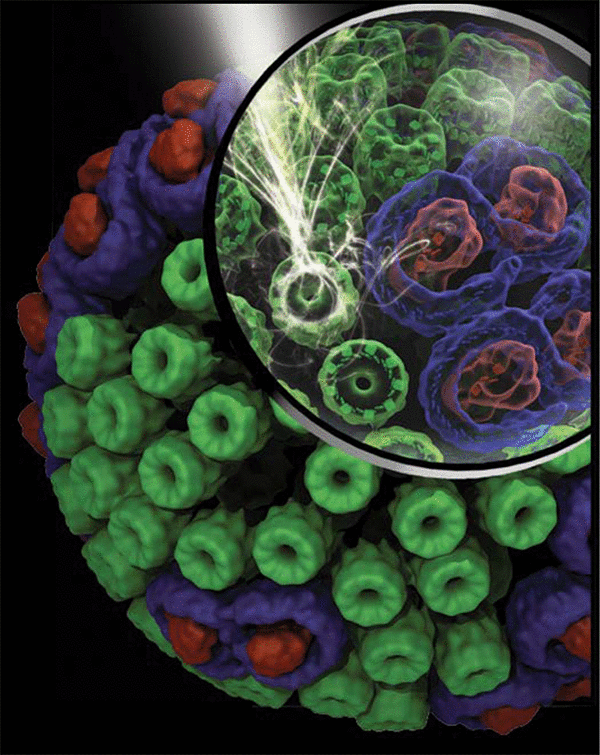
Structural model of the chromatophore.
Photosynthetic chromatophore vesicles found in purple bacteria are essentially biological solar cells built from light-harvesting proteins. Shown are over a hundred proteins, featuring approximately 3,000 bacteriochlorophylls, assembled in silico by combining AFM, NMR, EM, crystallography, spectroscopy, and mass spectrometry data.
A detailed structural exploration of the chromatophore, including lipids, can be found in this 3D/VR movie (article).
The energy conversion processes in the chromatophore from light absorption to ATP synthesis have been modeled in a recent manuscript, based on strutural model of the chromatophore.
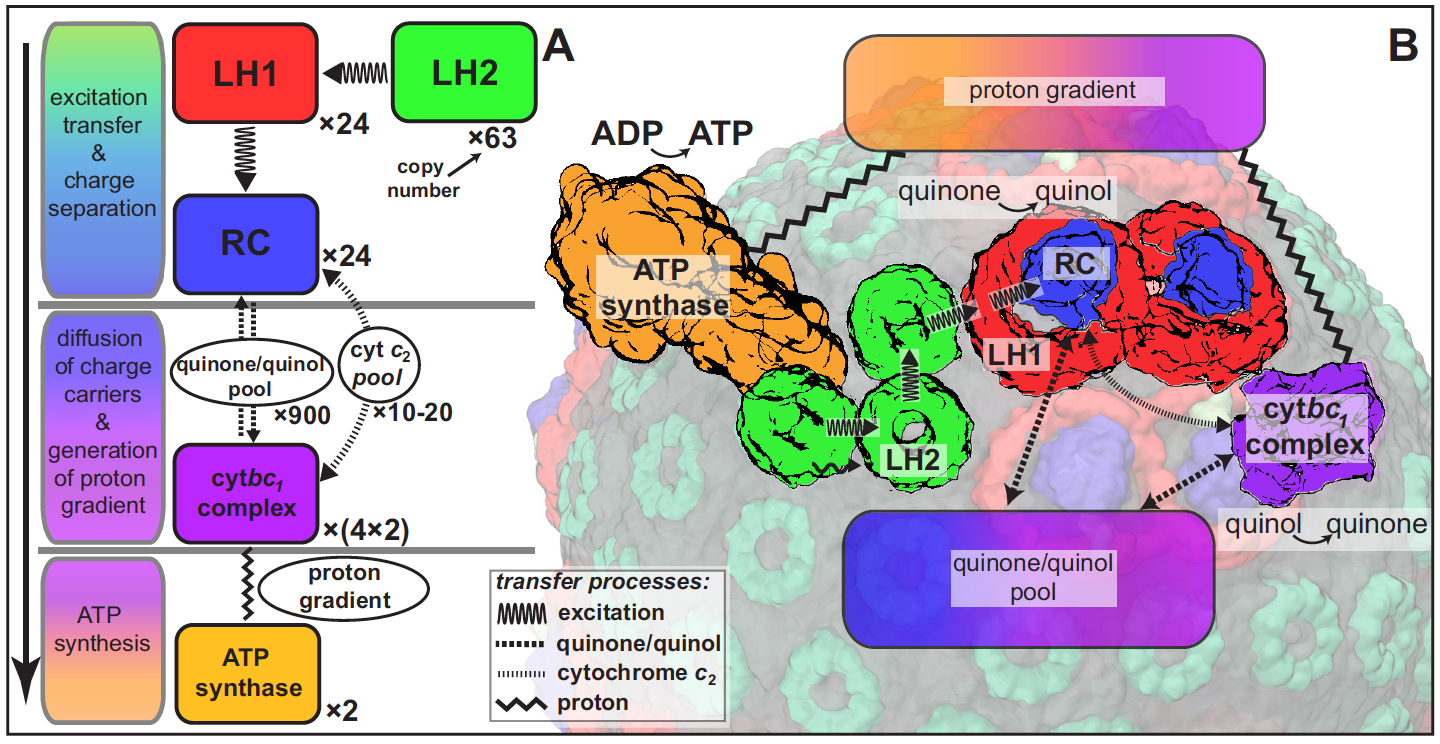
Energy conversion processes in the chromatophore.
Processes involved in energy conversion in the photosynthetic chromatophore. (A) Energy conversion processes starting after initial light
absorption are divided into three stages: (1) quinol production at RC as a result of excitation transfer; (2) diffusion between RC and cytbc1 of quinone/
quinol and cytochrome c2, together with quinol-to-quinone conversion resulting in a proton gradient across the vesicle membrane; (3) utilization of
proton gradient for ATP synthesis. (B) Chromatophore components, in which stages (1-3) take place, include LH2 (green), LH1(red)-RC(blue), cytbc1
(purple), and ATP synthase (brown) complexes as well as the lipid phase (olive).
These energy conversion processes have been visualized using high performance computing (article 1 and article 2) and presented in the form of a video, which has received the Best Movie award in the Supercomputing 2014 Visualization Showcase.
The stuctural and functional modeling of the chromatophore and the energy conversion processes therein enables to computation of the ATP production rate as well as the overall energy conversion efficiency of absorbed light energy into ATP.
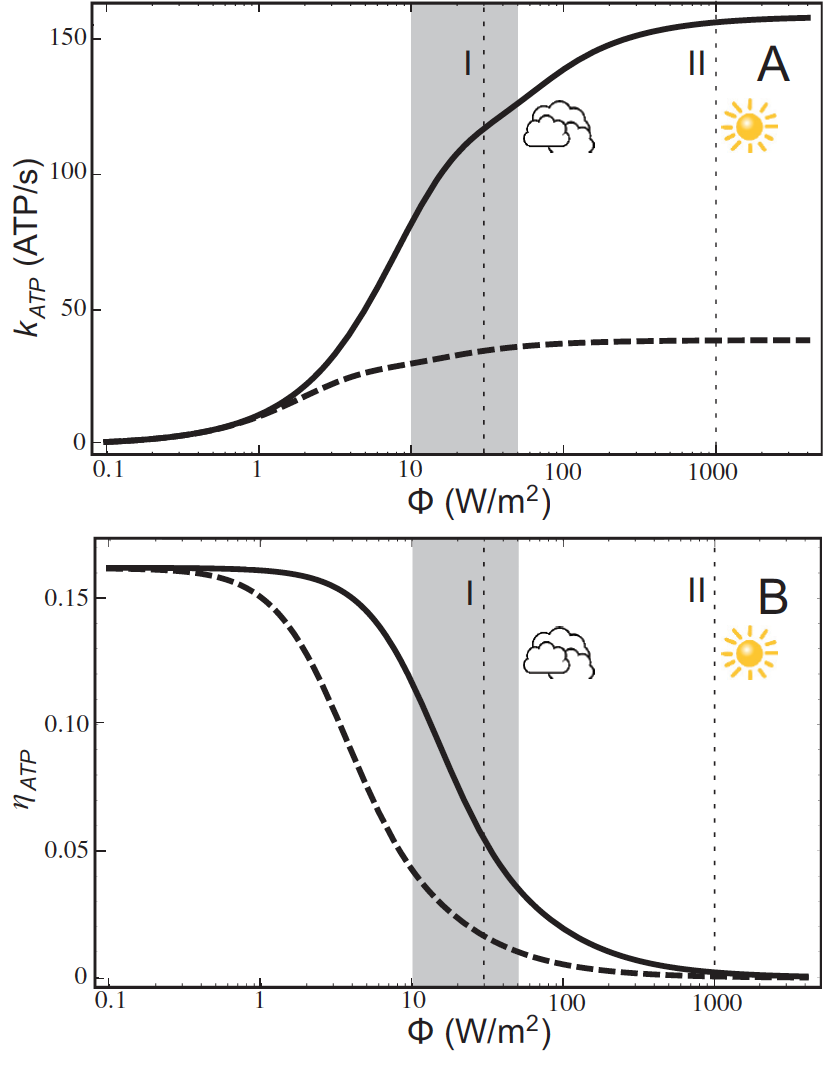
ATP production rate and energy conversion efficiency.
Steady-state ATP production rate (A) and energy conversion efficiency (B)
calculated
as a function of incident light intensity. Solid curves correspond to
the vesicle shown in the video above; dashed curves represent a similar vesicle with only a single cytochrome bc1 complex. The vertical lines
denote the light intensities corresponding to (I) 3% of full sunlight , a typical growth condition for purple
bacteria, and (II) full sunlight, respectively. Thus, for light intensities typical for the habitat of purple
bacteria (1-5% of full sunlight; shaded area) the energy conversion efficiency of a chromatophore vesicle is
between 0.12.-0.04.
Related web pages on our site
Chromatophore structure determination.A historical perspective on modeling photosynthesis.
Related publications
Overall energy conversion efficiency of a photosynthetic vesicle.
.
Melih Sener, Johan Strumpfer, Abhishek Singharoy, C. Neil Hunter, and Klaus Schulten.
eLife, 10.7554/eLife.09541, 2016. (30 pages).
Atomic detail visualization of photosynthetic membranes with GPU-accelerated ray tracing.
.
John E. Stone, Melih Sener, Kirby L. Vandivort, Angela Barragan, Abhishek Singharoy, Ivan Teo, Joao V. Ribeiro, Barry Isralewitz, Bo Liu, Boon Chong Goh, James C. Phillips, Craig MacGregor-Chatwin, Matthew P. Johnson, Lena F. Kourkoutis, C. Neil Hunter, and Klaus Schulten.
Parallel Computing, 55:17-27, 2016.
Visualization of energy conversion processes in a light harvesting organelle at atomic detail.
.
Melih Sener, John E. Stone, Angela Barragan, Abhishek Singharoy, Ivan Teo, Kirby L. Vandivort, Barry Isralewitz, Bo Liu, Boon Chong Goh, James C. Phillips, Lena F. Kourkoutis, C. Neil Hunter, and Klaus Schulten.
In Proceedings of the International Conference on High Performance Computing, Networking, Storage and Analysis, SC '14. IEEE Press, 2014. (4 pages).
Integration of energy and electron transfer processes in the photosynthetic membrane of Rhodobacter sphaeroides.
.
Michaël L. Cartron, John D. Olsen, Melih Sener, Philip J. Jackson, Amanda A. Brindley, Pu Qian, Mark J. Dickman, Graham J. Leggett, Klaus Schulten, and C. Neil Hunter.
Biochimica et Biophysica Acta - Bioenergetics, 1837:1769-1780, 2014
Atomic-level structural and functional model of
a bacterial photosynthetic membrane vesicle
.
Melih Sener, John D. Olsen, C. Neil Hunter, and Klaus Schulten.
PNAS, 104:15723, 2007.
People
