Highlights of our Work
2025 | 2024 | 2023 | 2022 | 2021 | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 | 2014 | 2013 | 2012 | 2011 | 2010 | 2009 | 2008 | 2007 | 2006 | 2005 | 2004 | 2003 | 2002 | 2001
Click to view the full-size image
Image size:
1.6MB
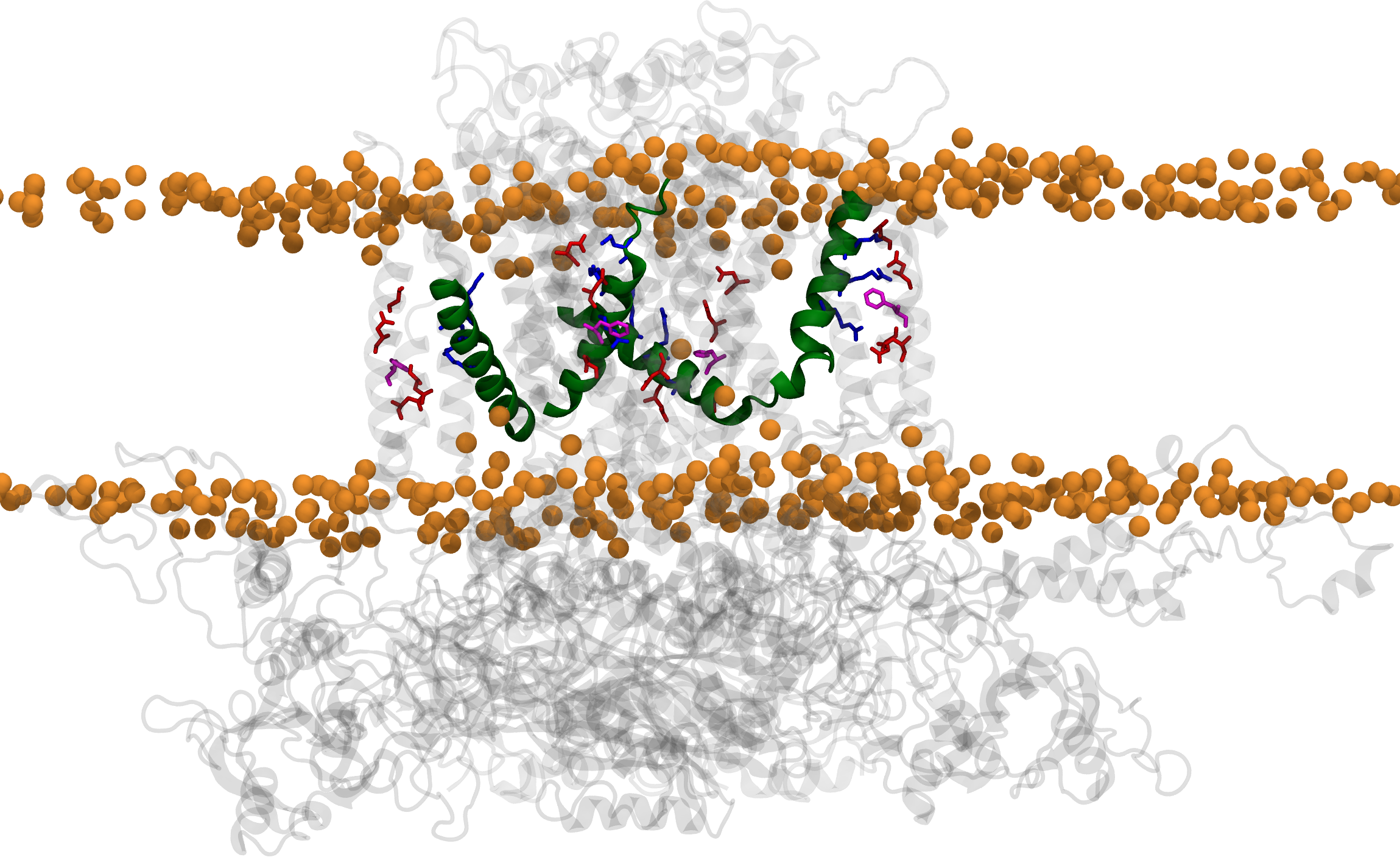
Made with VMD
The human hERG potassium channel is essential for maintaining normal cardiac rhythm. Mutations of hERG, particularly in its voltage‑sensing arginines, can lead to long‑QT syndrome, a condition characterized by potentially fatal arrhythmias. However, the structural basis of how these mutations impair the voltage sensing of the channel has remained elusive. In our recent Nature Communications study, we combined imaging and single‑molecule experiments with molecular dynamics simulations to capture the intermediate conformations of the voltage‑sensing domains. Simulations were visualized and analyzed using VMD, while simulations were run in NAMD. Our results reveal that disease‑linked mutations alter charge transfer within the sensor, hindering movement and thereby disrupting channel gating. These insights help explain the molecular basis of arrhythmias and suggest strategies to restore hERG channel function.



