Highlights of our Work
2026 | 2025 | 2024 | 2023 | 2022 | 2021 | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 | 2014 | 2013 | 2012 | 2011 | 2010 | 2009 | 2008 | 2007 | 2006 | 2005 | 2004 | 2003 | 2002 | 2001
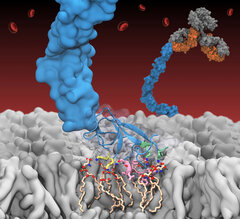
image size:
465.7KB
made with VMD
β2-glycoprotein I (β2GPI), a key autoantigen in antiphospholipid syndrome (APS), binds to negative lipids exposed on damaged cells, thereby enhancing its recognition by pathogenic antibodies. This membrane association—mediated by its fifth domain (DV)—is critical for disease progression, yet its molecular basis remains unresolved. As highlighted in our recent publication in J. Thromb. Haemost., Resource researchers employed molecular dynamics simulations using NAMD to investigate this key membrane binding event. Using an accelerated membrane model developed previously by the Resource, they captured membrane binding of DV and revealed how distinct interactions orchestrate its insertion into negative membranes. The study provides the first atomic-level insight into β2GPI’s membrane association and establishes a structural framework for novel therapeutics in APS.



