Light-harvesting in cyanobacterial photosystem I
|
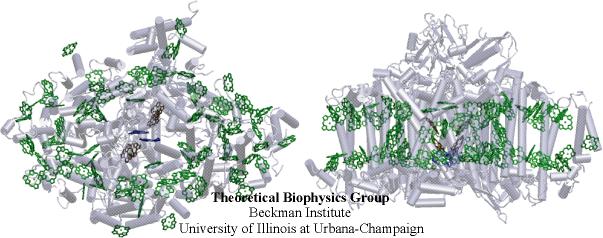
Photosystem I (PSI) is a highly ubiquitous pigment-protein complex used by green plants, cyanobacteria and algea to harvest the energy of sunlight. Most of the energy in our biosphere is supplied by molecular aggregates such as this one. Like most light-harvesting complexes PSI is a membrane protein comprised of a network of pigment antennae surrounding a reaction center. The antenna complex is responsible for the absorption of light and the transfer of the resulting electronic excitation to a so-called special pair of chlorophylls in the reaction center, where a charge separation across the cell membrane is initiated. This charge separation is in turn utilized for ATP synthesis.
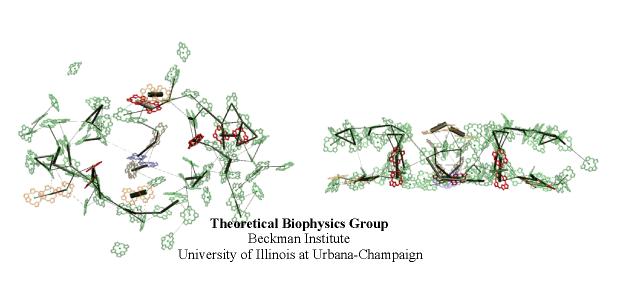
A high resolution (2.5 A) structure of PSI from the cyanobacterium Synechococcus elongatus based on x-ray crystallography was supplied by our collaborators [1]. This structure of PSI reveals 11 protein subunits, 96 chlorophylls, 22 carotenoids as well as other cofactors. Unlike the highly symmetric ring structure found in the photosynthetic apparatus of purple bacteria, the chlorophyll arrangement of PSI displays no obvious symmetries. Cyanobacterial PSI exists either as a trimer or a monomer as a function of external conditions such as light intensity. This structure opens new avenues along which the structure-function relationship in PSI can be elucidated. In a recent study [2], based on this structure, our group has investigated the light-harvesting function of this pigment-protein complex, based on an effective Hamiltonian formalism.
Our current interests include the study of the spectral and the energy transfer properties of the system. The effect of thermal disorder on the spectral properties is examined under the framework of random matrix theory and the excitation migration and trapping process is explored using Foerster theory. AcknowledgementThis material is based upon work supported by the National Science Foundation under Grant No. 0234938. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
References
|