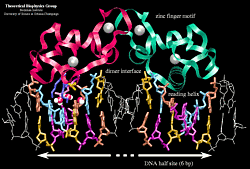
Estrogen Receptor Interacting with DNA
Nuclear hormone receptor proteins form a class of ligand activated proteins that, when bound to specific sequences of DNA serve as on-off switches for transcription within the cell nucleus. These switches control the development and differentiation of skin, bone and behavioral centers in the brain, as well as the continual regulation of reproductive tissues.
The nuclear hormone receptor proteins are composed of several domains which are differentially conserved between the various receptors and have different roles: a variable N-terminal region, a conserved DNA binding domain (DBD), a variable hinge region, a conserved ligand binding domain (LBD), and a variable C-terminal region.
The hormone receptor recognizes with markedly increased affinity a hormone response element (HRE). HRE is a specific DNA sequence that typically contains two consensus hexameric half-sites. The identity of a response element resides in three features: the sequence of the base pairs in the half-site, the number of base pairs between the half-sites and the relative orientation of the two half-sites. Thus each receptor protein dimer that binds the DNA has to recognize the sequence, spacing and orientation of the half-sites within their response element.
The goal of our study of the estrogen receptor interacting with specific and non-specifis sequences of DNA was to understand and explain what effect binding of ER has on the conformation of the target DNA, and how differences in the DNA sequence affect ER binding specificity
To answer these questions two systems were created:
- ER-ERE system based on the crystallographic structure of the ER-DBD bound to its consensus response element.
- ER-G/ERE system also based on the crystallographic structure but a non-consensus sequence of DNA was used instead of the consensus one. In this case, the first half-site corresponds to that of a consensus glucocorticoid response element.
The simulated complex of the estrogen receptor DNA binding domain dimer bound to DNA. The two mutated base-pairs in one half-site are highlighted. The sodium ions are shown as yellow vdw spheres and the water molecules as red dotted vdw spheres.
The picture was created with VMD.
All simulations were performed using the molecular dynamics program NAMD and version 22 of the CHARMm force field. NAMD provides the option of evaluating full electrostatic interactions through the use of a multipole expansion algorithm, namely the program DPMTA.
The results of our study show that the binding specificity of the ER-DBD to different sequences of DNA is correlated with how the side--chains of the protein position themselves relative to the bases of the DNA, most important being whether the side-chains can be accommodated without forming negative interactions with the bases. Also, while some hydrogen bonds are broken by the side-chains rearrangement in the non-consensus ER-G/ERE system, other bonds are created by the inclusion of water molecules. There is still an energy difference between the consensus and non-consensus complexes and that may arise from the less favorable geometry of the new hydrogen bonds. The water molecules found at the protein-DNA interface act favorably by filling gaps resulting from imperfect complementarity between interfaces and they also extend the interaction of some of the residues beyond their direct contact. Our simulations also demonstrate that interaction of the receptor dimer with DNA results in a bent and underwound conformation of the DNA compared to the initial crystallographic structure. The bend is described as a displacement or ``jog'' in the path of the helical axis when projected into a plane. We suggest that the induced bend in the DNA is a general effect of a hormone receptor dimer binding to DNA and that the bend reflects the symmetry of the dimer.
The binding of the estrogen receptor dimer to the DNA and the conformational changes induced in the axis of the DNA during the simulations are presented in an animated gif movie. Detailed results can be found in this poster.
Publications
Investigators
References
- B. F. Luisi, W. X. Xu, L. P. Freedman, K. R. Yamamoto, and P. B. Sigler. Crystallographic Analysis of the Interaction for the Glucocorticoid Receptor with DNA. Nature 352:497-505, 1991
- M. G. Parker, editor. Nuclear Hormone Receptors. Academic Press, Sad Diego, CA, 1991.
- Ann M. Nardulli and David J. Shapiro. Binding of the estrogen receptor DNA-binding domain to the estrogen response element induces DNA bending. Molecular and Cellular Biology, 12:2037-2042, 1992
- M. Sabbah, S. Le Ricousse, G. Redeuith and E. Baulieu. Estrogen Receptor-Induced Bending of the Xenopus Vitellogenin A_2 Gene Hormone Response Element. Biochemical and Biophysical Research Communications, 185(3):944-952, 1992
- Helmut Heller, Helmut Grubmüller, and Klaus Schulten. Molecular Dynamics Simulation on a Parallel Computer. Molecular Simulation, 5:133-165, 1990
- Brad Banko and Helmut Heller. User Manual for EGO - Release 1.1. Theoretical Biophysics Group at the University of Illinois at Urbana-Champaign, Beckman Institute, 405 N. Mathews Ave., Urbana, IL~61801, U.S.A., 1991. [Beckman Institute Technical Report TB-92-07]
- J. W. R. Schwabe, L. C. Chapman, J. T. Finch, D. Rhodes and D. Neuhaus. DNA recognition by the oestrogen receptor: from solution to the crystal. Structure 15:187-204, 1993
- Board, Jr., John A. and J. W. Causey and Leathrum, Jr., James F. and Andreas Windemuth and Klaus Schulten. Accelerated Molecular Dynamics Simulation with the Parallel Fast Multipole Algorithm. Chem. Phys. Lett. 198:89-94, 1992