Neurotransmitter Release Regulated by Synaptotagmin I
|
|
|
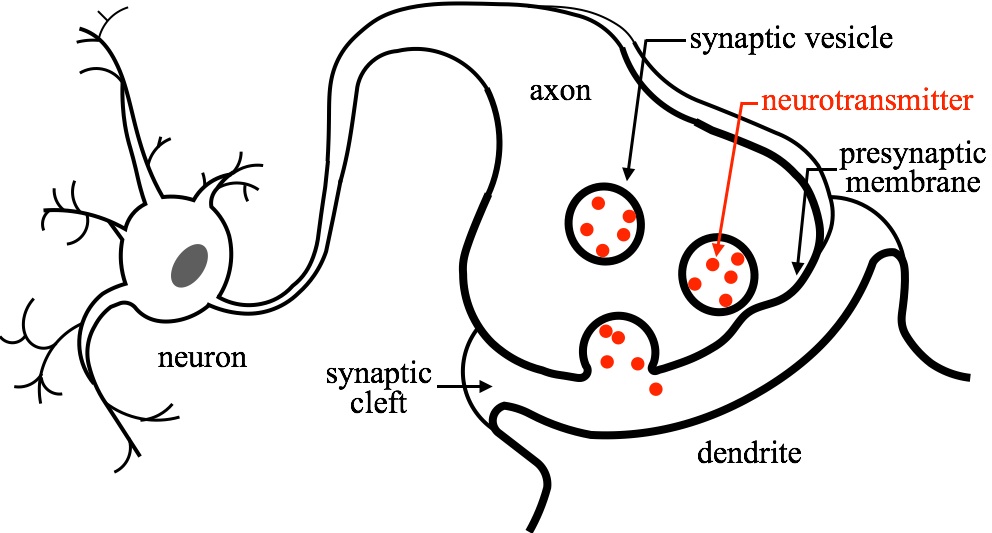
Fig. 1 - Illustration of the neurotransmission process. Neurotransmitters encasultated in vesicles are released to the synaptic cleft when the vesicles merge (fuse) with the presynaptic membrane. |
Overview
When an action potential propagates to a neuron axon terminal, calcium channels open and the local intracellular calcium ion concentration rises, triggering a membrane fusion event that joins the awaiting synaptic vesicles with the presynaptic membrane. Thus, neurotransmitters, stored within the vesicles, are released into the synaptic cleft through a fusion pore. Synaptotagmin I (syt1) has been identified as a calcium sensor protein that regulates, with the help of soluble n-ethylmaleimide-sensitive-factor attachment protein receptors (SNAREs), complexins, and other key proteins, membrane fusion in response to calcium concentration increase.
Despite intense efforts, the molecular mechanism of fusion between the vesicle and presynaptic membrane, and how syt1 regulates the fusion step, is far from clear. Molecular dynamics (MD) simulation, which has the power to study protein-ion-membrane interactions in atomic detail, can be a great tool to investigate the fusion process.
Synaptotagmin causes membrane cruvature, a key step in fusion
|
|
|
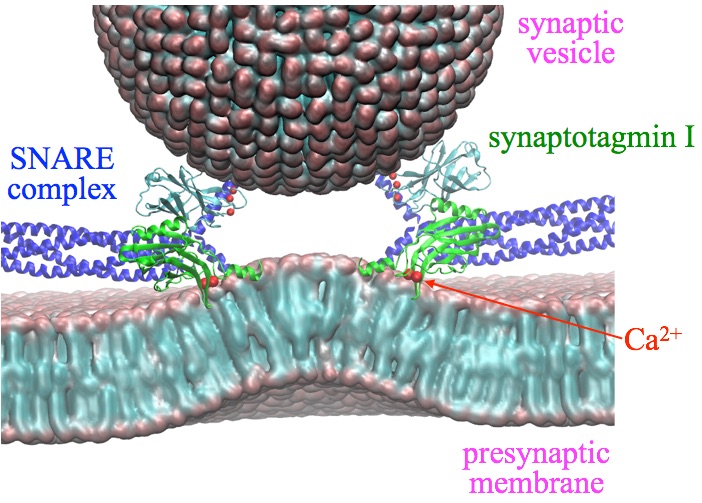
Fig. 2 - Illustration of synapse and the key proteins. The presynaptic membrane is bended by synaptotagmin I, which is a key step towards membrane fusion between vesicle and presynaptic membrane. |
It has been shown that the Calcium bound synaptotagmin 1 induces presynaptic membrane bending, which can be essential to fusion pore formation. If syt1's membrane-bending activity is abolished, it does not regulate membrane fusion anymore. Therefore, it is essential to study how syt1 induces membrane bending, a key step towards membrane fusion.
Mechanistic insight into syt1's membrane bending can be obtained from comparing the two calcium binding domains in syt1, namely, C2A and C2B. Despite the domains' similarity in structure, sequence, and membrane binding affinity with bound Calcium, isolated C2B can bend membranes effectively, but isolated C2A cannot. The most obvious difference between the C2A and C2B domains is that the C-terminal helix arises only in C2B. Because this helix (409VEEEVDAMLAVKK421) has many hydrophobic and positive lysine residues that can interact with the negatively charged membrane, as well as negative residues that are close to the calcium-loop, the C-terminal helix might generate membrane curvature upon calcium binding.
Synaptotagmin conformational transition is required to trigger membrane bending
|
|
|
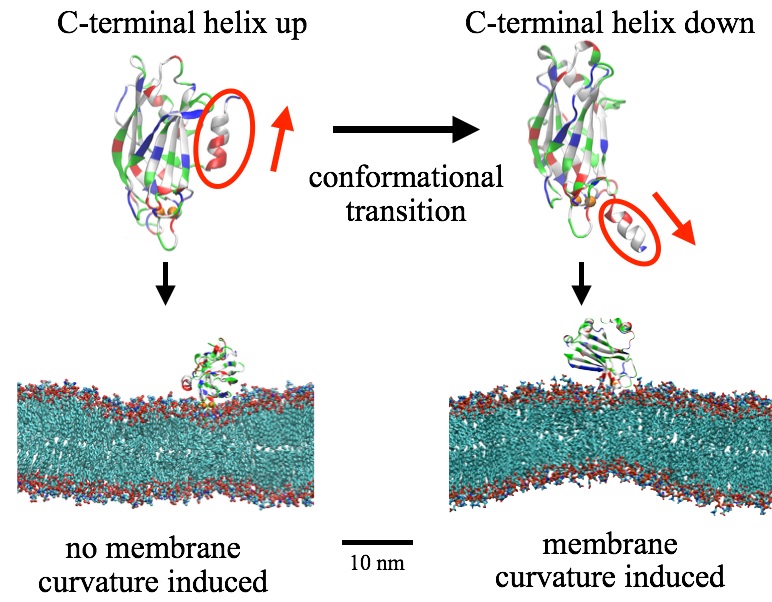
Fig. 3 - Conformational transition in synaptotagmin C2B's C-terminal helix triggers membrane bending. |
Based on computer simulations, the authors suggest that a key detail of the available structures: the C-terminal helix in the C2B domain reorient upon binding to both calcium and membrane. Membrane bending arises only after the helix alters its orientation from pointing away (up) from the membrane surface to an orientation pointing toward the membrane (down). In such down-orientation, the negatively charged residues in the helix interacts with the calcium ion in the calcium loop, while the hydrophobic and positive residues makes contact with lipids and causes membrane curvature.
With the C-terminal helix in the helix-down conformation, C2B-induced membrane bending occurred spontaneously in the simulation. The bent membrane adopted curvature within ~100 ns, characterized through a ~102-nm diameter curve that was maintained throughout the rest of the simulation (1000 ns).
When C2B's C-terminal helix is truncated, the bent membrane will be flatened, demonstrating the necessity of such helix in membrane bending. Moreover, when the calcium ions are removed, the whole C2B domain (including the C-terminal helix) is not stable on membrane. The calcium ions anchor syt1 on membrane and facilitate helix-membrane interaction, and thus, regulate membrane bending and fusion.
Synaptotagmin-induced membrane bending facilitates membrane fusion
|
|
|
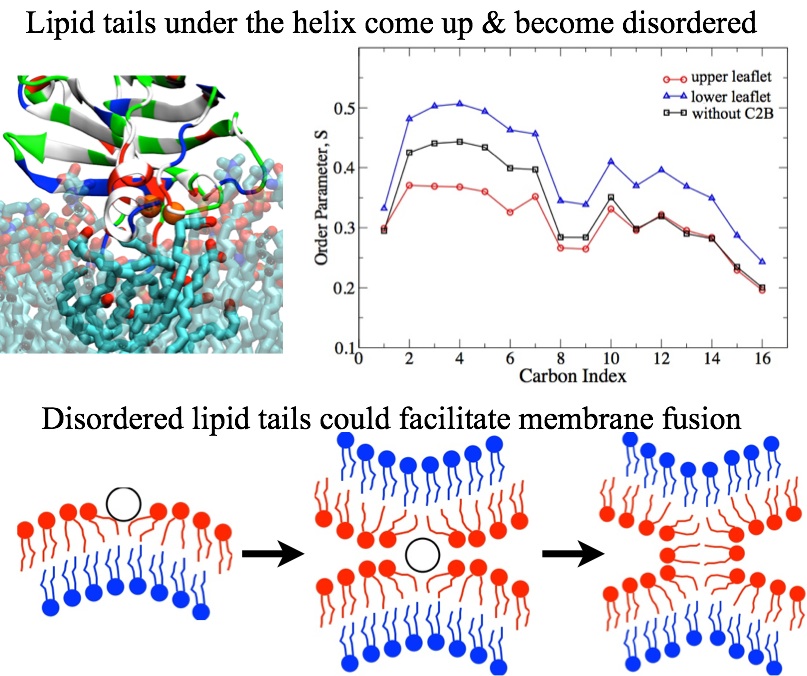
Fig. 4 - The C-terminal helix in C2B domain causes lipid tail reordering that differs significantly between the membrane bilayer leaflets. Such lipid tail structure triggers membrane bending and eventually facilitates membrane fusion. |
The synaptotagmin C2B domain bends membrane with its C-terminal helix shallowly inserting in membrane. Such insertion causes strong reordering of lipid tails that differs significantly between the membrane bilayer leaflet proximal to the C2B domain and the one distal. Lipids in the proximal leaflet move their tails towards the membrane surface, exposing them to the C-terminal helix hydrophobic residues and solvent; lipid tails in the distal leaflet instead strainghten up to fill the void left in the proximal leaflet and become more ordered. The different ordering results in an increase in lateral pressure in the proximal leaflet and a decrease in the distal leaflect, and results in membrane bending.
Hypothesis have been voiced that solvent-exposed lipid tails facilitate membrane fusion. Indeed, several fusion peptides, such as melittin, influenza hemagglutinin, and their mutants, interact with lipid tails of the proximal membrane leaflet and drive them to the membrane surface. One may conclude, therefore, that solvent-exposed lipid tails can facilitate the fusion of two membrane without lipid headgroup dehydration. Accordingly, the solvent-exposed lipid tails induced by syt1's C-terminal helix may drive both membrane bending and membrane fusion, which explains the experimentally observed correlation between the two membrane remodeling processes.
Related publications
Synaptotagmin's role in neurotransmitter release likely involves Ca2+-induced conformational transition
Zhe Wu, Klaus Schulten.
Biophys. J. 107:1156-1166 (2014)
Investigators
Support
Center for the Physics of Living Cells
Page created and maintained by Zhe Wu.



