Novel Energy Transduction in P-glycoprotein
P-glycoprotein - a multidrug resistance transporter
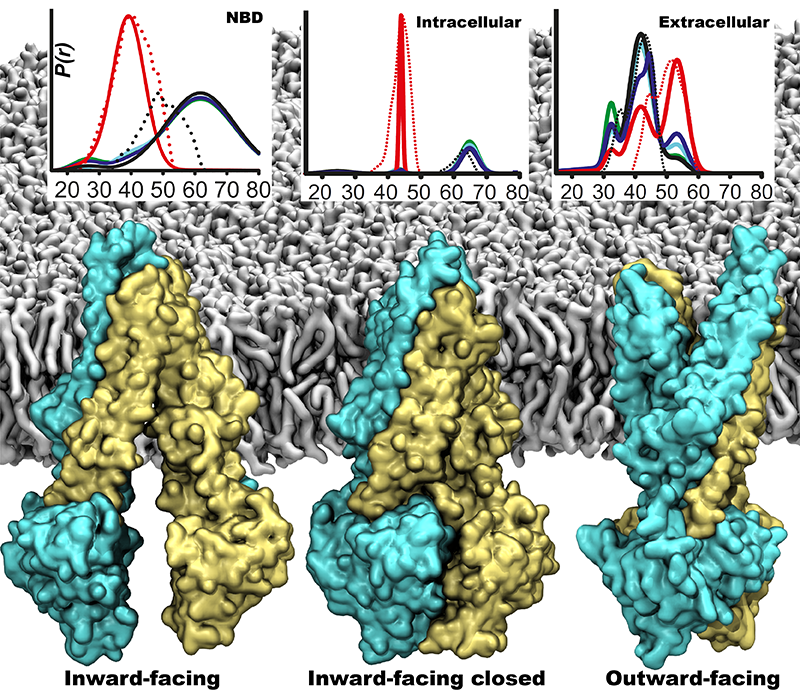
Fig. 1 Major conformational states of P-glycoprotein during its large-scale IF to OF transition. Representative plots obtained from DEER experiments are showing the OF P-gp conformations are heterogeneous compared to its IF conformations
P-glycoprotein (P-gp) is a membrane transporter known to transport a wide array of small molecules from amino acids to drugs. Unlike its prokaryotic homologs, P-gp is a single polypeptide chain folds into two pseudo-symmetric halves. Each half is composed of six transmembrane (TM) helices and a remote intracellular nucleotide-binding domain (NBD). Nucleotide binding and hydrolysis at NBDs drive the large-scale structural transition in which P-gp shuttles between its inward-facing (IF) and outward-facing (OF) states (Fig. 1). During this transition, the substrate is transported out of the cell. A major rearrangement of TM helices also occurs during this large-scale transition, which makes the OF state of P-gp look much different from its IF state. All the P-gp crystal structures available so far are solved in IF state and thus a reliable OF model is of high significance.
In our recent study published in Nature, we constructed a reliable model for OF state of P-gp using a hybrid approach combining homology modeling and non-equilibrium molecular dynamics simulations [1]. Our independent computational predictions matched very well with the experimental observations performed by our collaborators from Vanderbilt University and University of Virginia.
Modeling the Unknown OF state
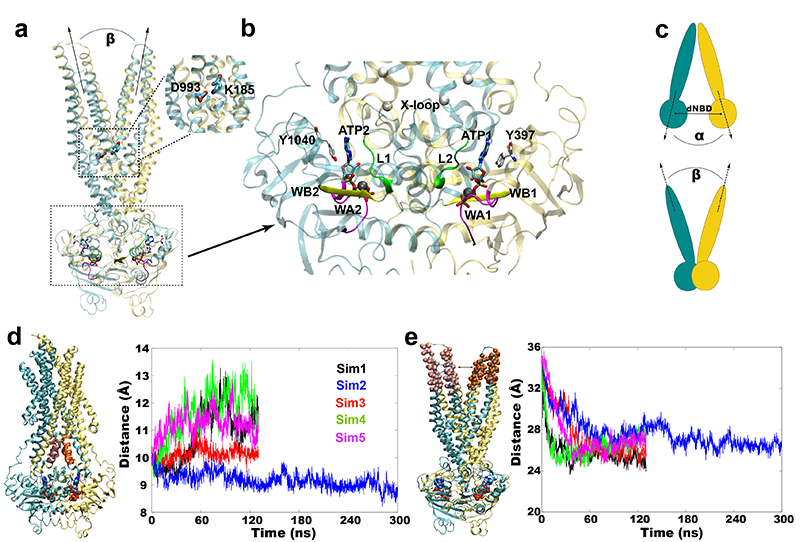
First part of the Homology modeling of OF state of P-gp was based on structure-based sequence alignment between P-gp and MsbA, a bacterial homolog, as a structural template (PDB ID. 3B60). The initial homology model was further refined using the IF crystal structure of P-gp (PDB ID. 4M1M) to reconstruct the regions that did not have any structural equivalence from the bacterial homolog followed by docking of ATP molecules [2]. The complete homology model, after docking nucleotides into the NBDs, was embedded in a lipid bilayer and subjected to MD simulations. Within 10 ns of MD simulations, the model displayed a closing behavior at the extracellular side. This could be due to the incorrect modeling of NBD dimer interface, which composes of more intricate molecular interactions, which could not have formed by simple docking of nucleotides into NBDs. Thus we employed a 30 ns non-equilibrium MD simulation to form essential nucleotide binding and NBD-NBD dimerizing interactions to form the reliable dimer. Until the dimerization of NBDs occurred, we have restrained the extracellular opening of the OF state (Fig. 2). A salt bridge interaction at the level of head groups of the membrane lower leaflet was also formed enabling the tight closure of the intracellular side of TM domain. Finally, we performed a 300 ns MD simulation and the model was stably closed at the intracellular side and open at the extracellular side. Compared to the stable intracellular side, extracellular side was observed to be flexible. Experimental observations also have shown a similar trend as extracellular being heterogeneous in the observed distance distributions (Fig. 1). This conformational flexibility at the extracellular side could arise from a relatively large number of proline and glycine residues in the TM regions, as we suggested previously [2].
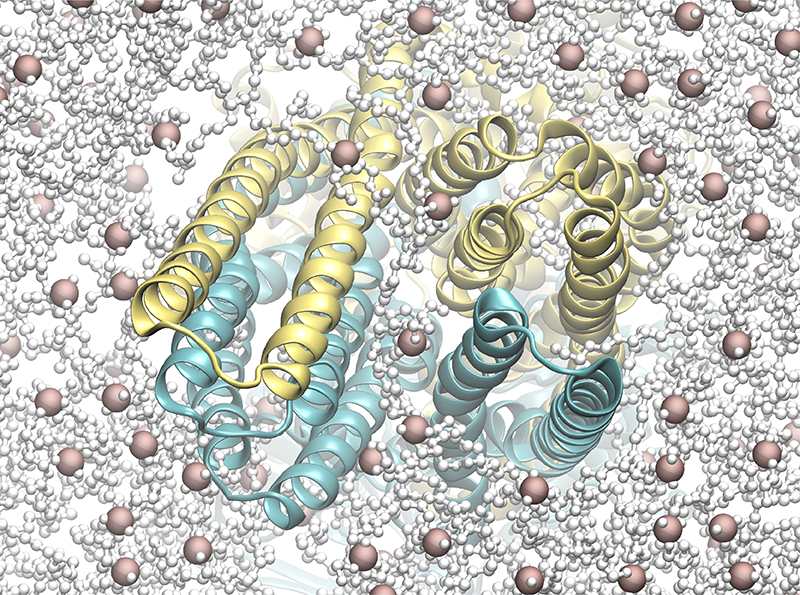
Lipid Dynamics Stabilize the OF state
Performed MD simulations also uncovered an unprecedented detail regarding the stability of the OF state of P-gp. Lipid tails inserted at the extracellular opening of P-gp during the restrain stage of the simulation [3] (Fig. 3). Another key characteristic important for the stabilization of the OF state, which was completely unexplored thus far. These inserted lipid tails may facilitate the exit of the substrate exported by P-gp.
Related Publication
-
Energy Transduction and Alternating Access of the mammalian ABC Transporter P-glycoprotein. B. Verhalen, R. Dastvan, S. Thangapandian, Y. Peskova, H. A. Koteiche, R. K. Nakamoto, E. Tajkhorshid, and H. S. Mchaourab. Nature, 2017. DOI: 10.1038/nature21414
-
On the Origin of Large Flexibility of P-glycoprotein in the Inward-Facing State. P.-C. Wen, B. Verhalen, S. Wilkens, H. Mchaourab, and E. Tajkhorshid. Journal of Biological Chemistry, 2013. DOI: 10.1074/jbc.M113.450114
-
The cellular membrane as a mediator for small molecule interaction with membrane proteins. C. G. Mayne, M. J. Arcario, P. Mahinthichaichan, J. L. Baylon, J. V. Vermaas, L. Navidpour, P.-C. Wen, S. Thangapandian and E. Tajkhorshid. Biochimica et Biophysica Acta (BBA) - Biomembranes, 2016. DOI: 10.1016/j.bbamem.2016.04.016
Investigators
Page created and maintained by Mrinal Shekhar.



