Research Topics - Bioenergetics
Photosynthetic complexes provide fascinating challenges to biophysicists. With the availability of recent atomic structures for protein-pigment complexes such as photosystem I in cyanobacteria and plants, it is possible to form a comprehensive picture of the light absorption and excitation migration processes based on an atomic level quantum mechanical description. This kind of structural analysis not only provides a rigorous test for our understanding of the physics of these mechanisms through a comparison to spectroscopy and kinetics experiments, but it also forms a framework within which the organizational principles for multi-component pigment-protein assemblies can be investigated.
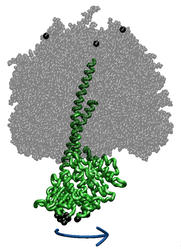
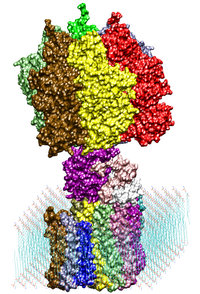
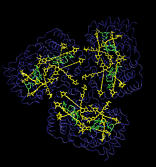
ATP synthase is a large multi-protein complex which includes a transmembrane Fo unit coupled to a solvent-exposed F1 unit via a central stalk. The stalk rotates within the surrounding subunits of F1, leading to cyclic conformational changes in the three catalytic sites in F1 and, thereby, to ATP synthesis. We use steered molecular dynamics to apply a torque to the central stalk in order to understand the cooperative interactions that underlie this mechanism.
Adenosine triphosphate (ATP) is the primary energy "currency" in most living organisms. ATP synthase is a large (about 100,000 atoms) protein, which includes a transmembrane F0 unit coupled to a solvent-exposed F1 unit via a central stalk gamma. The F0 unit utilizes a transmembrane electrochemical potential (proton motive force), converting it into the mechanical energy of the stalk rotation. The rotation leads to cyclic conformational changes in the catalytic sites in the F1 unit, thereby driving ATP synthesis. We seek to identify and explore the chain of the elementary chemical (proton transfer) and mechanical (domain motion) events involved in the process of converting the electrochemical energy of the transmembrane proton gradient into the mechanical energy of the c subunit oligomer rotation.
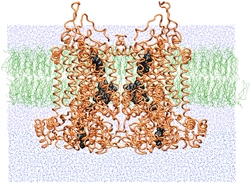
SMD simulations provide insight into how the bc1 complex performs its function of separating electrons and protons across a membrane.
Light harvesting complexes provide fascinating challenges to biophysicists. With the availability of atomic structures for protein-pigment complexes such as photosystem I, it is possible to form a comprehensive picture of the light absorption and excitation migration processes based on an atomic level quantum mechanical description. This kind of structural analysis not only forms a rigorous test for our understanding of the physics of these mechanisms through a comparison to spectroscopy and kinetics experiments, but it also provides a framework within which the organizational principles for multi-component pigment-protein assemblies can be investigated.
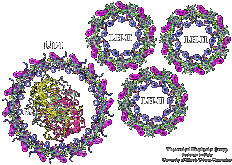
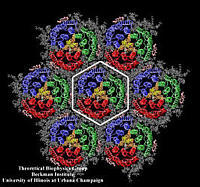
Photosynthetic units (PSUs) of living organisms are complex assemblies of several multi-protein aggregates. We constructed the structure of the PSU of purple bacteria. The components involved were the crystal structure of the bacterial photosynthetic reaction center surrounded by the model structure of the light-harvesting complex I and several structures of the light-harvesting complex II, solved in a collaborative project with X-ray crystallographers. The constructed structure is used to study the light energy transfer driving the bacterial photosynthesis.
The purple membrane (PM) of halobacterium salinarium consists of bacteriorhodopsin trimers, lipids and water molecules. Upon light absorption by the chromophore inside bacteriorhodopsin, protons are pumped across the PM to the extra-cellular side of the membrane. With molecular dynamics simulations of the whole PM, we are studying the influence of the native environment on the protein dynamics.
In light-harvesting complexes, carotenoids act as light-absorbers in the blue-green region of the spectrum. Absorption of a photon is followed by rapid singlet excitation energy transfer to bacteriochlorophyll (BChl). In addition to their light-harvesting role, carotenoids photoprotect antenna complexes, i.e., they prevent the formation of photo-oxidizing singlet oxygen by quenching BChl triplet states through triplet excitation transfer. Light-harvesting and photoprotection by carotenoids is studied in Theoretical Biophysics Group in two proteins, the Light-Harvesting Complex II of Purple Bacteria, and the Peridinin-Chlorophyll-Protein of Dinoflagellates.