Nucleotide Transportation by the ADP/ATP Carrier
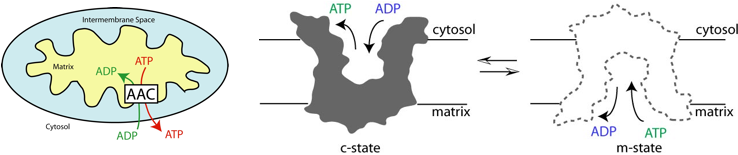
Adenosine triphosphate (ATP) is the fuel of a cell. In animal cells, ATP is synthesized by joining an adenosine diphosphate (ADP) with an inorganic phosphate inside the mitochondria. ATP is then exported to the cytosol where its hydrolysis releases the energy stored in the molecule. On the other hand, ADP, the product of ATP hydrolysis, is shuttled back into mitochondria for the next round of ATP production. The transportation of ADP and ATP across the mitochondrial membrane is mediated by a membrane protein named ADP/ATP carrier (AAC). In an exchange cycle, AAC switches between a cytosolic-open state (c-state), to which ADP binds from the cytoplasm, and a matrix-open state (m-state), where ATP binds from the mitochondria. The only available crystal structure of AAC is that of the c-state in complex with an inhibitor. As a result, precise knowledge of the ADP binding site inside the carrier lumen remained unkown.
| In a recent study, we performed extensive molecular dynamics simulations of the c-state AAC, investigating the ADP binding process and probing its binding site. Our simulations revealed rapid, spontaneous binding of ADP to deeply positioned binding sites within the carrier lumen. The ADP binding process revealed by one of these simulations is shown in the movie on the left. The figure on the right shows the snapshots of ADP binding revealed by a second simulation. These simulations are the first example of a complete ligand binding event described in full atomic detail using unbiased simulations. Putative ADP binding sites are revealed, which are found to be buried deeply inside the lumen of AAC. Electrostatic analysis of trajectories shows the presence of an unusually strong positive electrostatic potential in the lumen (the blue mesh in the figure), which constitues the main driving force for the observed spontaneous binding of ADP. | 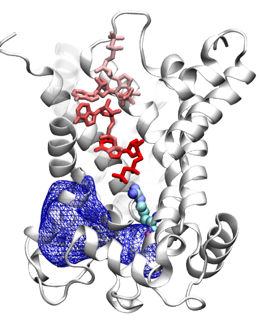
|
Further analysis indicates that the positive electrostatic potential is likely a common attribute among the entire family of mitochondrial carriers. The positive potential may play a key role in the substrate recruitment and translocation, as well as in anchoring the carriers into the negatively charged inner mitochondrial membrane.
Using steered molecular dynamics, we also probed the conformational changes of the carrier during nucleotide translocation. These simulations reveal that through rearrangement of salt bridges at the bottom of the lumen, ADP binding and translocation can induce outward displacements of three transmembrane helices of the protein. The rearrangement of salt bridges ``unlocks'' these helices, allowing it to expand in the matrix half. These results shed light on key structural elements and initial steps involved in conversion of the carrier from the c-state to m-state.



