Computational Biology of the Aquaporin Water Channels
The image below illustrates the results of computer simulation of an aquaporin, a membrane protein discovered and investigated by Peter Agre, winner of the 2003 Nobel Prize on Chemistry. The simulations represent one of the largest and longest simulations ever for a membrane channel in its natural environment, namely embedded in the cell membrane (bright red) and immersed in water (blue). The simulated system involves more than 100,000 atoms for which interactions and motions have been calculated 5,000,000 times. The calculations were possible only due to the recent availability of a large cluster of fast computers at the NSF National Supercomputing Centers in Pittsburgh and Urbana.
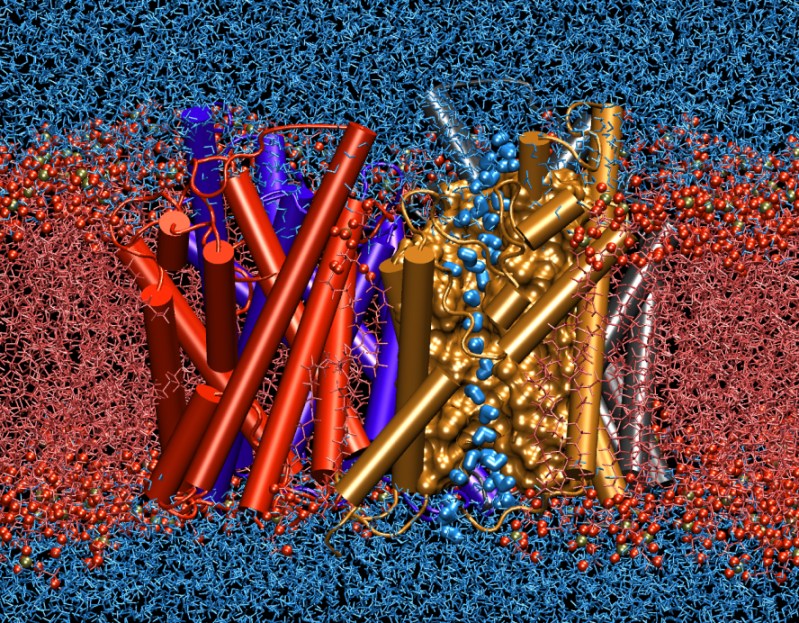
The simulated protein is one of the membrane water channels, called aquaporins, which are responsible for efficient transport of water across oily cell membranes. These proteins are present in all forms of life, including bacteria, plants, and mammalians, and play critical roles in control of water in different organs. In the human body more than ten different types of them have been found. They are present in all human organs that need to either react quickly to osmotic changes in their environment, or deal with translocation of large volumes of water. In our kidneys, for example, they are in charge of of concentration of urine through reabsorption of more than a bathtub (200-250 liters) of water every day. Impaired function of these channels are associated with several common diseases, such as diabetes insipidus and congenital cataracts.
Although each single protein provides an independent conduit for water transport, water channels form tetramers (sets of four) in the membrane. These proteins are shown in cartoon representations and using four different colors in the image. The computer simulations of these proteins that were conducted at the NIH Resource for Macromolecular Modeling and Bioinformatics at the University of Illinois at Urbana-Champaign are a prime example of success of computer modeling of biological problems. They could not only for the first time simulate completely the transport of materials across the membrane in full detail, but also resulted in predictions about the structure and function of the channels that had puzzled molecular biologists for many years. The results of this study, which appeared in the April 2002 issue of Science magazine (1), provided such a deep understanding of how the channels function so fast but very selectively.
The flow of water molecules through the channel is mediated by simultaneous displacement of water molecules that were found in simulations to form a single file inside the channel, as shown in the figure for one of the four channels by highlighting water molecules with a different representation. Very interestingly, during their passage at the center of the channel water molecules are forced to rotate 180 degreed before they are allowed to continue. This tuning mechanism, which was discovered and described at an atomic level by the simulations, turned out to be the basis of an extremely important selectivity mechanism of the channel, and prevents the discharge of electrical charge of the membrane, which is one of the main energy storage means in living cells.
Visit our main aquaporin research page for more information.



