Research Projects - Quantum Biology
Many important biological processes taking place in cells are driven and controlled by events that involve electronic degrees of freedom and, therefore, require a quantum mechanical description. An important example are enzymatically catalyzed, cellular biochemical reactions. Here, bond breaking and bond formation events are intimately tied to changes in the electronic degrees of freedom. Key events during photosynthesis in plants and energy metabolism in eucaryotes also warrant a quantum mechanical description - from the absorption of light in the form of photons by the photosynthetic apparatus to electron transfer processes sustaining the electrochemical membrane potential. Because of the importance of sensing light to both plants (for regulating vital functions) and animals (for vision), the interaction between light and biological photoreceptors is widespread in nature, and also requires a quantum mechanical description. A prime example is the protein rhodopsin which is present in the retina of the human eye and plays a key role in vision. Our computational tool are combined quantum mechanical/molecular (QM/MM) simulations, that allow to combine an electronic level description of the active region with a classical model of the environment provided by the remainder of the biomolecular system and solvent. This allows us to study the electronic level processes underlying these systems in their natural cellular environment.
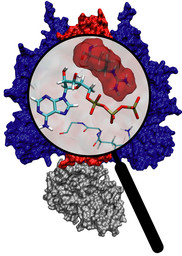
image size:
202.7KB
made with VMD
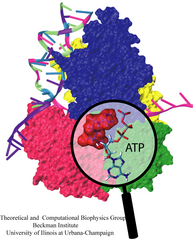
Molecular motors are efficient nanoscale machines destined to make any human designed engine look clumsy. F1-ATPase is such a machine - so powerful that a spoonful of it could produce as much torque as your car's engine. As part of the enzyme ATP synthase, the protein can work as an engine but also operate in reverse as a generator. In the latter mode it is responsible for the synthesis of the energy-rich molecule ATP that serves as fuel driving many processes in biological cells. It can also convert the energy stored in ATP into mechanical rotation. Our studies suggests that the analogy to a car's engine goes even further! A quantum chemical description of the reaction of ATP combined with a simulation of the protein revealed that an amino acid side group of the protein, called the "arginine finger", controls the progression of the catalytic event, much like a spark plug controls the combustion process in a car engine. The very extensive simulation made use of a powerful computer, the Jonas Cluster at the Pittsburgh Supercomputing Center. The investigation is yet another example for the important role of computational biology unraveling the secret behind the function of the machinery of living cells.
Light harvesting complexes provide fascinating challenges to biophysicists. With the availability of atomic structures for protein-pigment complexes such as photosystem I, it is possible to form a comprehensive picture of the light absorption and excitation migration processes based on an atomic level quantum mechanical description. This kind of structural analysis not only forms a rigorous test for our understanding of the physics of these mechanisms through a comparison to spectroscopy and kinetics experiments, but it also provides a framework within which the organizational principles for multi-component pigment-protein assemblies can be investigated.
In light-harvesting complexes, carotenoids act as light-absorbers in the blue-green region of the spectrum. Absorption of a photon is followed by rapid singlet excitation energy transfer to bacteriochlorophyll (BChl). In addition to their light-harvesting role, carotenoids photoprotect antenna complexes, i.e., they prevent the formation of photo-oxidizing singlet oxygen by quenching BChl triplet states through triplet excitation transfer. Light-harvesting and photoprotection by carotenoids is studied in Theoretical Biophysics Group in two proteins, the Light-Harvesting Complex II of Purple Bacteria, and the Peridinin-Chlorophyll-Protein of Dinoflagellates.
image size:
162.1KB
made with VMD
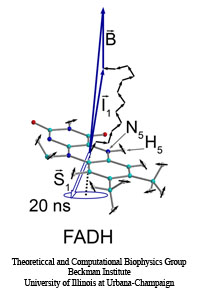
Plants and other photosynthetic organisms convert sunlight into various forms of metabolic energy. To expose themselves optimally to the sun while at the same time avoiding damaging overexposure to light, these organisms employ molecular photosensing systems that control, for example, the orientation of their leaves. Common photosensing systems include photoreceptors of the so-called phot family that are sensitive to blue light and contain Light, Oxygen, and Voltage (LOV) sensitive protein domains as photoactive elements. Light absorbed by a flavin molecule leads to bond formation with the protein (LOV domain), thereby, initiating signaling until the flavin-protein bond breaks spontaneously. Our study of the photosensing events in the LOV domain of the algae C. reinhardtii employs computer simulations that combined quantum mechanical and classical simulation methods to study photoexcitation and subsequent processes. It emerged that formation of the flavin-protein bond is initiated by a unique light-driven transfer of a hydrogen atom between the LOV domain and the flavin molecule.
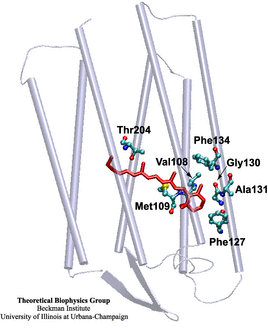
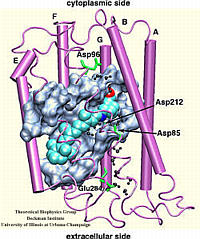
The rhodopsin receptors reside in the cell membrane, and function as sensors of light. These proteins consist of an apoprotein (opsin) and a retinal chromophore covalently bound to the apoprotein by a protonated Schiff base linkage to a lysine residue. While the protonated form of retinal Schiff base absorbs at about 440 nm in organic solvents, its maximal absorption is drastically changed after binding to the apoprotein, an effect known as 'opsin shift'. A fundamental challenge in vision research has been the elucidation of the physical mechanisms by which the protein matrix adjusts the maximal absorption of the chromophore, using the molecule retinal to detect light at different wavelengths. The spectral tuning in two very homologous rhodopsins, sensory rhodopsin II and bacteriorhodopsin, is investigated by means of a combined ab initio quantum mechanical/molecular mechanical calculation.
The all-trans retinal protonated Schiff base (RSPB) is the chromophore of bacteriorhodopsin (bR), a transmembrane protein that acts as a light-driven proton pump in Halobacterium salinarium, converting light energy to a proton gradient. Upon absorption of light the chromophore undergoes a photoisomerization process (all-trans -> 13-cis) that eventually provides the driving force for the translocation of protons. This elementary photoisomerization process proceeds on multiple coupled potential energy surfaces and we have modeled it using a formally exact quantum-mechanical procedure: the full multiple spawning method. Currently, we are studying the first excited electronic state of the chromophore using an isolated retinal analog model and ab initio CASSCF methods. The characterization of the first excited state (minima and conical intersections associated with isomerization around different double bonds) will enable us to extend and improve the aforementioned quantum-mechanical studies of the photoreaction dynamics in the protein.
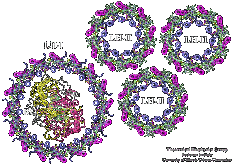
Photosynthetic units (PSUs) of living organisms are complex assemblies of several multi-protein aggregates. We constructed the structure of the PSU of purple bacteria. The components involved were the crystal structure of the bacterial photosynthetic reaction center surrounded by the model structure of the light-harvesting complex I and several structures of the light-harvesting complex II, solved in a collaborative project with X-ray crystallographers. The constructed structure is used to study the light energy transfer driving the bacterial photosynthesis.
image size:
1.9MB
made with VMD
image size:
231.6KB
made with VMD
Excitation transfer between pigment molecules, such as bacteriochorophylls (BChls), and between pigment-protein light harvesting complexes, such as light harvesting complex 2 (LH2), has been investigated for many years using many different theoretical descriptions. Typically these descriptions include a priori assumptions about the dynamics of the system. Such assumptions are often made due to incomplete knowledge to make the system numerically tractable so that further insight can be gained. In the theoretical models of excitation transfer, it is often assumed that one parameter is much larger than another, allowing the system to be treated perturbatively. These assumptions, however, should be physically reasonable and should be tested if possible.
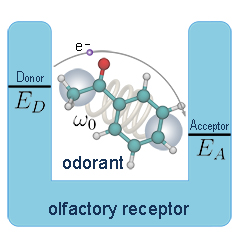
Olfaction is the sense of smell. This sense is mediated by specialized sensory cells of the nasal cavity of vertebrates. Many vertebrates, including most mammals and reptiles, have two distinct olfactory systems—the main olfactory system, and the accessory olfactory system (used mainly to detect pheromones). For air-breathing animals, the main olfactory system detects volatile chemicals, and the accessory olfactory system detects fluid-phase chemicals. Olfaction, along with taste, is a form of chemoreception. The chemicals themselves that activate the olfactory system, in general at very low concentrations, are called odorants. Volatile small molecule odorants, non-volatile proteins, and non-volatile hydrocarbons may all produce olfactory sensations. Some animal species are able to smell carbon dioxide in minute concentrations.
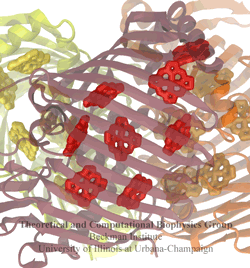
image size:
218.3KB
made with VMD
Green sulphur bacteria harvest sunlight by absorbing solar energy in large pigment-containing vesicles known as a chlorosomes and transporting this energy to a reaction center for charge separation. Along the way, excitation absorbed by the chlorosome is passed through the Fenna-Matthews-Olson (FMO) complex to get to the reaction center complex. As this process occurs in a biological environment, there is a significant amount of thermal noise present. As excitation is passed between pigments, from the bacteriochlorophylls in the chlorosome to those in FMO and finally to those in the reaction center, it is constantly under the influence of thermal fluctuations. It is of importance then that FMO can efficiently conduct excitation energy, i.e., without much energy loss. One way to do this could be by exploiting quantum coherence to speed up energy transfer.
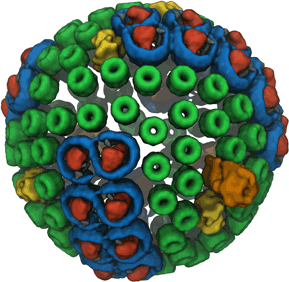
image size:
740.7KB
made with VMD
Photosynthesis, the main source of energy for all life, is performed by an intricate assembly of hundreds of proteins, which harvest, transfer, convert, and store solar energy. The simplest such light harvesting machine is the purple bacterial photosynthetic unit (PSU), which performs anoxygenic photosynthesis and is significantly simpler and evolutionarily more primitive than its counterpart found in plants. The bacterial PSU is organized in the form of a pseudo-spherical membrane domain of approximately 60 nm diameter called a chromatophore vesicle.
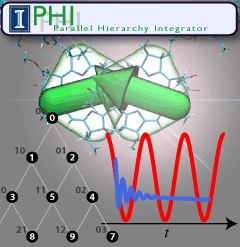
Quantum mechanics rules all natural processes, but is manifested most strongly when acting on the lightest particles, namely the well-known electrons. To study quantum effects physicists routinely resort to very low temperature, that of liquid helium. Amazingly, living systems seem to exploit quantum effects for their benefit, but do so at temperatures typical for life, namely around room temperature or warmer. A particularly important case is photosynthetic light harvesting where so-called quantum coherence plays a critical role when electrons in assemblies of chlorophylls become excited by sun light and the excitation energy is harvested by utilizing it to charge photosynthetic membranes. In order to understand how photosynthesis can exploit room temperature quantum effects one needs to know how the temperatures, which are much higher than those in the physics laboratories where liquid helium is employed for cooling, affect electron behavior. The knowledge can be gained by so-called dissipative quantum mechanical descriptions, but the needed computer calculations are extremely demanding. To address this demand, researchers have developed the software PHI that uses the power of parallel computers, as described here. PHI has already been used to understand how many chlorophyll molecules act together to absorb sunlight among themselves and let the excitation migrate between chlorophylls to so-called reaction centers where the excitation energy is converted into a membrane potential. The PHI software can be obtained from our web site. More information on PHI is available here.