Historical Series: Photosynthesis
Unraveling Photosynthesis Step by Step:
Four Decades of Research in Theoretical and Computational Biophysics
By Lisa Pollack
September 2012
Revised July 2013
Revised November 2014
See other related articles by L. Pollack
Early Influences on Klaus Schulten
“Today you think it's exciting, but in those days it was considered the most boring thing you could do, to study molecules,” remarks Klaus Schulten on his decision in 1969 to pursue a PhD in molecular physics. “That was done in the 1930s, and even then it was boring.” But Schulten had a master plan, and he surmised molecular physics was the right field to pursue in graduate school, never tempted by the discoveries promised in the hot new field of particle physics. He wanted to study biology, but specifically at the level of atoms and molecules, and he imagined that physics would give him the tools he needed to accomplish this.
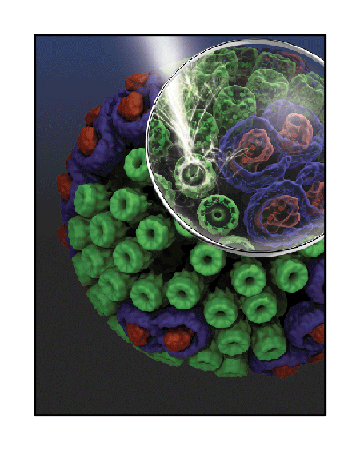
The chromatophore, a compact cellular compartment, as envisioned by Klaus Schulten's research group.
From a very early age Schulten was fascinated by biology. A voracious reader in all areas of science, he discovered, around age twelve, Du und das Leben (You and Life), a book by the eminent biologist Karl von Frisch, and the contents captivated him. “He was a great biologist who very early on appreciated the molecular basis of living systems. So he brought the two together and wrote on what life is, but that it comes from the molecules.” In fact, there was one particular chapter that caught his attention, about how cells employ photosynthesis to make ATP, the molecule that fuels life. “I felt that was my calling,” Schulten says; “I wanted to understand how life forms use energy to make ATP and then run many processes with it.”
Through high school Schulten continued to read on his own about science, finding very little stimulating on it in the classroom. In fact, because he liked to challenge his teachers with the things he taught himself, he was given the nickname “Professor” by his classmates. “When I got my degree at the high school, the teachers asked me, what do you want to study, and I said I want to study it all: chemistry, physics, mathematics, and biology. And that, of course, was further proof for them that I was totally crazy.”
When Schulten was in his early twenties, studying for his diploma at the University of Münster, he often walked in its famed botanical garden with fellow students. “I discussed at length with my friends what I wanted to do with my life, and that is exactly what I do today.” But what he wanted to do didn't exist at the time–he wanted to look inside living cells, at the level of electrons and atoms, to understand how they functioned; and he wanted to do it theoretically instead of experimentally.
The story charted in the following pages will reveal how a life's calling was realized over the course of forty-plus years. In its beginning stages, a tapestry of people in Schulten's early adulthood, when woven together, led to a familiarity and then a fascination with photosynthesis. Despite this fascination, Schulten at one point had to wait ten years before he could do the kinds of calculations he deemed worthy of publication: “What I really like about the photosynthesis is it goes from the molecule, even the electron, to the whole cell. And that is basically what I always wanted to do; that is why I stuck with it for a long time.”
Instead of plants, Schulten chose to study purple bacteria, which embody the simplest form of photosynthesis. Bacteria emerged around 3.5 billion years ago on the earth; photosynthesis, an ancient process, arose sometime thereafter, although no firm evidence exists to prove its exact origins. While many associate it with plants, photosynthesis most likely originated in bacteria, and photosynthesis is found in many different phyla of bacteria today, purple bacteria being one of them. The purple bacteria are a gift for scientists studying photosynthesis, as the machine or unit that runs photosynthesis in these bacteria is simpler than the ones in plants.
Image from Du und das Leben, a book that captivated the young Klaus Schulten.
Even in its least complicated incarnation, Schulten never dreamt that he could understand thoroughly this phenomenon when he started the field in the 1970s. But forty years of dedication has paid off. “At the end, today,” summarizes Schulten, “I think we are pretty close to really going full circle and understanding everything.” This history documents how the pieces all fell together and, after setbacks and dry-spells, resulted in an impressive compendium of work by many scientists from different fields who worked with Schulten on a topic so key for life on earth.
Graduate School at Harvard: The Invisible States
When Schulten finished his diploma in physics at Münster, he carefully considered his next step, one that would bring him closer to his childhood dream. As a youth he had a mathematical mind and a love for living systems. At the school aquarium he wanted to understand the fish and the plants, and why they were alive. “I was never interested in just a feather, or in a bone,” he recounts about his youth. “It was more like this living entity that all comes together, that's what interested me.” When deciding on graduate schools, he asked around and the only person at the time who combined physics with living systems was Martin Karplus at Harvard.
Formally trained as a theoretical chemist, having worked with Linus Pauling at Cal Tech in graduate school, Martin Karplus spent many years doing chemistry-related research before he returned to his first love, biology. In the fall of 1969 he took a leave of absence from his position as a chemistry professor at Harvard to visit the Weizmann Institute in Israel to uncover how the tools of a theoretical chemist could be applied to biological problems. He came back from the institute with a host of problems in biology to study, his stay a success.
Around this same time, Klaus Schulten decided he wanted to work with Karplus on his PhD, for he knew Karplus combined mathematics, physics, chemistry and biology in his research, a long-held goal for Schulten. With a fellowship from the Volkswagen Foundation, Schulten began his studies, and would eventually receive a degree in chemical physics.
One of the areas of study Karplus chose to pursue after his stay in Israel was the role of retinal in vision. Retinal is related to the carotenoids, for basically half a carotenoid molecule is vitamin A, and when oxidized vitamin A becomes retinal. Retinal also is one example of a polyene, a type of chemical compound with multiple carbon-carbon bonds. Polyenes play key roles in light absorption in vision as well as in photosynthesis. Studying polyenes would become a major focus of the Karplus group at Harvard.

Martin and Marci Karplus, circa 1976.
Photo used with permission.
Karplus, in his various jobs over the years, always appreciated the fresh perspectives he gained from talking to the other colleagues at his institution. One of the fuels that fed Karplus's polyene research came from interactions with a new assistant professor in chemistry, Bryan Kohler, whose experiments intrigued Karplus. With his graduate student Bruce Hudson, Kohler had found that when they shined light on certain polyenes and looked at the resulting excitation bands, there was a little excitation they couldn't explain. The wisdom at the time held that there was some dirt in the solution they couldn't clean away, which was responsible for this weak absorption. “So then they cleaned and cleaned but it didn't go away,” recalls Schulten about this mystery. “And they went to my advisor, Karplus, and asked him if there is a chance that there is some electronic excitation there. I was just a new student of Karplus's, and so when you have a new student you also have a new victim.”
Karplus and Schulten decided to employ the so-called electron correlation effect to see what would happen if they excited a pair of electrons in the polyene in a coordinated manner. “You might say if you lift two electrons up that's crazy,” says Schulten about this theoretical approach. “That costs twice as much energy, but that's not necessarily so. And because the electrons might do it in such a coordinated way that the excitation is actually lower in energy than moving just a single one, that is what I was going to try out.”
Schulten started this work in the fall of 1971, and actually stayed in Boston over the subsequent winter break to investigate this new problem and prove the existence of forbidden, low-lying states. He vividly remembers a snowstorm during that time, which almost paralyzed the region; Schulten was barely able to get out and buy food. But that was not his greatest concern: “I was always worried that Kohler and Hudson would come through the door any moment and tell me, 'Oh, sorry, we found a better cleaning agent and now the effect is gone.'”
Because it was winter break, Schulten had the office and computer center all to himself. “I worked there day and night,” Schulten recalls. “Basically I remember that I only stopped working when my hand got cramped from holding the pen, because I had to derive all these quantum mechanical rules that describe the system.”
Schulten was very careful, however, and even did the calculations two or three times to make sure he made no mistakes. His diligence paid off; when Karplus returned from break, Schulten showed him proof of the mysterious low-lying states. “These other states were not seen because they are what physicists would call magnetic,” explains Schulten. “They actually involve the spins, and spins are basically magnetic moments.” His work revealed that polyenes have certain triplet-triplet excitations responsible for the feeble excitations the experimentalists witnessed. (A triplet has two electrons whose total spin adds to unity.) “If you take two triplet excitations in the polyene and add them up,” spells out Schulten, “you are getting an excitation energy that is still lower than the lowest excitation that is seen in an optical transition.”
|
"WHEN YOU HAVE A NEW STUDENT YOU ALSO HAVE A NEW VICTIM." Klaus Schulten |
Publishing this new finding, however, was not straightforward. Other researchers claimed that what Kohler and Hudson saw was a well-known “dirt effect” and not something real. They actually claimed the dirt was in the window of the cuvette and not in the polyenes themselves. Schulten, a rookie in computational biology at that point, summarizes how the paper was likely received by some in the scientific community: “Do you know what foolish thing Karplus did? He wrote a paper on this dirt effect.” But Schulten and Karplus persevered and eventually published their findings. Schulten's understanding of these polyene excitations would later inform his photosynthesis research.
While Schulten was applying techniques of theoretical chemistry and physics to biological problems in his formal studies, his life outside the classroom at Harvard was enriching his mind just as much, and helping to shape his future research interests. He loved to spend free time in the biology labs of George Wald and Ruth Hubbard, a power couple on campus. “Several times they threatened to kick me out,” recalls Schulten, “because I was too arrogant. I was a physicist, and so I always thought I was so much smarter than all the biologists.” George Wald received the Nobel Prize in 1967 for having discovered that retinal is used by all species with eyesight. Vision, like photosynthesis, is a process involving the interaction of biomolecules with light and Schulten learned about retinal and the carotenoids from Wald. “He was a real big influence on me,” Schulten muses; “I was interested in vision but then I knew of course that these kind of molecules also play a role in photosynthesis.”
The Boston area around Harvard was a vibrant environment, especially for young scientists. While living there Schulten also heard about some work on carotenoids by the Tufts biochemist Norman Krinsky. Known later as the father of modern carotenoid research, one of the many things Krinsky worked on was studying the protective role of carotenoids. Although carotenoids are pigments that absorb light, Schulten heard about research that proposed another role for the carotenoids: as quenchers of excited oxygen. Krinsky and many others working in the field in the 1960s and 1970s found that the length of carotenoids was related to their protective function, and that mutant strains with shorter-than-normal lengths would not exhibit protection for the bacteria; that is, bacteria with shorter carotenoids would die when exposed to light. Schulten grew fascinated upon hearing about this other role for carotenoids, as protectors of living organisms.
Max Planck Institute for Biophysical Chemistry
After finishing his PhD, Schulten returned to Germany to work at the Max Planck Institute for Biophysical Chemistry in Göttingen. Dozens of different Max Planck Institutes exist all over Germany, conducting innovative research in many fields of science and the humanities. Albert Weller, an institute director in Göttingen who hired Schulten, ran an experimental group focused on characterizing electron transfer reactions. Weller immediately gave Schulten the job of working out an oddity in one of Weller's pet reactions, namely why something called “fast triplets” were observed. In this reaction the scientists saw a mysterious product that was an excited molecule with a pair of electrons whose spins were parallel, hence a triplet. The product occurred faster than normally expected.Schulten got to work straightaway. “I figured out where it comes from,” he explains. “And I also figured out that you could verify it by doing the reaction with and without magnetic fields.” What Schulten essentially discovered is that a magnetic field can influence a chemical reaction, a new physical effect. He immediately published an initial, qualitative paper suggesting the effect, and then, teaming up with his wife, Zan Luthey-Schulten, and Hans-Joachim Werner, a student in the group that Schulten was mentoring, they presented the full quantitative theory of the effect in two papers published in 1977.

Albert Weller, a director of the Max Planck Institute for Biophysical Chemistry from 1971-1990. Image taken from here.
Because the computing facilities at Göttingen were not stellar, the three had to use sophisticated mathematical tricks for this mammoth feat. “We achieved a numerical solution,” recounts Schulten, “of the problem of how two molecules that transfer electrons between them, and carry their unpaired electron spins that move around and back, how they can react with each other in a solvent.”
Around 2010 Schulten found himself refereeing a paper for Nature by a well-known physics group, and was surprised to discover that they presented exactly the same computations that the three theorists at the Max Planck Institute had painstakingly carried out over three decades earlier, without the benefit of sophisticated computers. As Schulten sees it, “something that people were proud of in 2010 we did in 1976.” But Schulten and his collaborators were not satisfied with just detailing the qualitative theory behind this new magnetic field effect. They began to think about all the ways it could be applied.
In electron transfer, an electron moves from one place to another, a seemingly simple process. But Weller and collaborators had worked for many years fine-tuning a system of donor and acceptor molecules that was optimal for making measurements in the laboratory. While Weller's pet system, pyrene and dimethylaniline, was a chemical system, the scientists who worked for Weller at the institute were fully aware that electron transfer was an important process in biological systems as well. Since Schulten knew about electron transfer in biological systems, he started thinking about application of the new magnetic field effect to biological systems, ones beyond the chemical systems favored in Weller's group. “Basically, when you have a new physical effect,” notes Schulten, “like this magnetic field effect, you very often can use it as a measuring stick.” But what kind of measuring stick is optimal? “The measuring sticks that you usually are looking for are ones that observe processes that are too miniscule in terms of size, and too fast in terms of time, to observe easily in experiment.” In other words, all one needed to do was put the magnetic field on the system, then take it away, and see if there was a different outcome.
Electron transfer processes were known to be involved in photosynthesis. Being in an electron transfer group, Schulten says that in the back of everybody's minds was an awareness of just how important electron transfer was to photosynthesis. So in 1978 Schulten and Werner, along with their director, Albert Weller, published a paper suggesting that the new effect could be used as a ruler to characterize in more detail the electron transfer that occurs in photosynthesis. The goal was to use the magnetic field effect to understand better the electron transfer in photosynthesis.
There were many reasons why Schulten already knew enough about electron transfer in photosynthesis to suggest using the magnetic field effect as a yardstick in his 1978 paper. The electron transfer in photosynthesis occurred in a protein called the photosynthetic reaction center, which may be thought of as the heart of the photosynthetic unit. When plants and bacteria absorb energy from the sun, this energy is used by the reaction center to transfer an electron. The workings of the reaction center were not fully understood because the structure of this protein was not known in the late 1970s when Schulten was in Göttingen. “So people actually did very well,” recalls Schulten, “to conclude, from certain optical properties and from electron transfer rates, what the structure looked like. So it was amazingly good actually, but it was not firm.”
One of the groups that did, however, study these electron transfer reactions, despite the uncertainty about the reaction center structure, was located in the Netherlands, in Leiden. Schulten knew them and their work quite well. Led by Arnold Hoff, they studied magnetic resonance in photosynthesis, and Schulten was impressed by how far they got without knowing the exact structure of the reaction center. When Schulten published the 1978 paper suggesting that the new magnetic field effect be used as a yardstick to characterize electron transfer in photosynthesis, Albert Weller, his boss, was one of the co-authors. Referring to this paper, Arnold Hoff told Schulten that Hoff should have been an author on the publication too. “Now Hoff didn't do anything,” recounts Schulten, “so he didn't have a reason to be an author of this paper. But he said that Weller had even less of a reason.” Schulten did not buy this argument, as he felt that Weller ran a great institute that inspired and enriched him as a scientist. “People ignore a little bit the role that the big guys play,” says Schulten. “They not only give you a seat to sit on, they also give you ideas. They sell the whole of science by saying why it is important, and what will be done in the next few years. If they wouldn't do that, one couldn't do one's work.”

Klaus Schulten, in his office in 1978 at the Max Planck Institute in Göttingen.
While Schulten knew about the Leiden group and their work, another reason he was aware of current photosynthesis research he attributes to being in Germany. “Science has strong local aspects,” he explains, “so that certain feats of science are done more in one country than another, for example. And photosynthesis was studied a lot in Germany.” One of those hubs was located in the Technical University of Berlin, at the Max-Volmer Institute for Physical Chemistry, which was really put on the map by Horst Witt. Schulten visited the institute often and learned a great deal about Witt's efforts, which focused on explaining oxygen-generating photosynthesis. “He was very bold,” Schulten notes. “He did it actually before one knew much, but he really struggled hard to give a picture of what could be involved. And that really also gave me a broader picture of photosynthesis as a biological process that involves many different steps and many different proteins. That motivated me to understand how living cells actually combine many steps to have a good outcome.”
Besides the groups in Berlin and Leiden, Göttingen also shaped Schulten's views on the complexity of biological processes that would later inform his own photosynthesis work. One of those Göttingen influences was a man who worked there at the institute, Manfred Eigen. In 1967 Eigen received the Nobel Prize in chemistry for having studied very fast reactions. Although trained in physics and chemistry, his attention turned to biological processes later in his career, especially to the origin of life. “Here we have the molecules,” Schulten says of Eigen's views on biological organization, “and here we have the whole cell: surviving, producing enough energy for its survival, and doing so many levels of reactions. And to begin to understand the challenges involved in organizing all those levels was really the program of Eigen's intellectual life in a way.” This influenced Schulten to always consider biological organization in a living cell.
While Schulten went to lectures by Eigen in Göttingen, he also participated in the famous annual gathering known as the Winter Seminar, in Klosters, Switzerland. There Schulten learned to ski during the days and listened to cutting-edge talks at night. Started in 1965, this gathering first encompassed only coworkers at the institute. In an online video, Manfred Eigen discusses how the Winter Seminar originated: “We should go away from the lab, because in the lab you have your daily life here and you should really go to some nice silent place. And since there are so many meetings in summertime, you say, let's have a winter seminar and let's discuss there our problems. And if we have time left, let's go skiing.” Later the seminar grew in popularity and attracted an international following; Eigen estimates that between 30 and 50 Nobel Prize winners attended in the course of its run over several decades. The seminar was unique, as it purposely did not require an abstract and manuscript and completely finished work, as most meetings did at the time. “In other words,” continues Eigen in a second video, “people are uninhibited to talk about things because they are not pinned down. They can speculate in the seminar, and that makes it lively.” Being near a force like Manfred Eigen, who wanted to understand biological complexity, encouraged Schulten to think of photosynthesis as an overall process that, although it involves many steps, should be viewed as a unit.
Göttingen also brought Schulten's attention to a critical equation by physical chemist Theodor Förster, via his supervisor Albert Weller. Schulten knew about the various accomplishments of German Theodor Förster because Weller had worked with Förster for many years studying protolytic reactions in the 1950s. From 1946 to 1949 Förster published three papers that described how an excited molecule transfers its excitation to an acceptor molecule. Soon becoming known as Förster Resonance Energy Transfer, or FRET, this mechanism now made it possible to describe how one chlorophyll transfers its excitation to a nearby chlorophyll during photosynthesis. “Förster made a tremendous contribution to science with it, way beyond photosynthesis,” remarks Schulten on this equation. “His formula and his idea are used today all over the world in many laboratories.”
While the accomplishments of Förster and his famous equation came to Schulten naturally through Weller's lineage to Förster, Schulten is quick to point out that Förster was not the only one in the 1940s to wonder at the mechanism of excitation transfer between pigments in photosynthesis. The famous physicist Robert Oppenheimer, known for his work on the Manhattan Project during World War II, published in the minutes of the American Physical Society a brief abstract in 1941 entitled “Internal Conversion in Photosynthesis,” right before he got caught up with the war effort. In the abstract he addresses excitation transfer from certain pigments (namely, fluorescent dyes) to chlorophylls in photosynthetic algae, suggesting the transfer is a large-scale model of internal conversion of gamma rays. After the war, in a 1950 paper with William Arnold, the pair elaborated on the mechanism of excitation transfer between chlorophyll and phycocyanin, a pigment used as a dye. William Arnold had a background in physics and biology and had worked on experimental aspects of photosynthesis since the 1930s. Arnold had done some work to show that light absorbed by the pigment phycocyanin was transferred to chlorophylls instead of directly reducing carbon dioxide. When he told Oppenheimer about this finding, they set out to explain the mechanism of excitation transfer. While the pair discussed this idea of excitation transfer as internal conversion sometime around 1939 or 1940, they did not get around to publishing this explicitly until 1950, according to Arnold's autobiographical article.
|
"GOETTINGEN WAS THE PLACE WHERE MY SCIENTIFIC DIRECTION WAS SHAPED" Klaus Schulten |
But why is the Oppenheimer and Arnold work still relatively unknown? Schulten notes that their abstract approach was not particularly helpful for biologists. “Förster was more of a physical chemist, not a physicist, and he had a much better way of explaining what he learned,” says Schulten, about the difference between Förster's and Oppenheimer's strategies. “In particular Förster described very carefully the elementary act of sharing the excitation between two chlorophylls. Oppenheimer knew that, too, but then he immediately averaged over hundreds of them. But what was really very important later was to know the pair behavior.”
Although Schulten published his first paper ever on photosynthesis in 1978 while at the Max Planck Institute, his next foray into the field would come only after a ten-year hiatus. But the serendipitous opportunity that presented itself a decade later would prove a treasure trove for a computational biophysicist like Schulten. The reason for the hiatus was because Schulten realized that for his theoretical purposes, the data was just not there–the structure of the reaction center was still unknown. “One could have done more,” says Schulten, “but it would have always been questioned.”
Living Among Experimentalists
From 1974 to 1980 Schulten worked in Göttingen, and although he only published one paper, among many, on photosynthesis, his time at the Max Planck Institute was critical to his future work in the field. “Göttingen was the place where I learned the most and where my scientific direction was shaped most,” Schulten notes. “And that was clearly due to the fact that I lived among experimentalists.”
Schulten was surrounded by 40 or 50 experimentalists who worked in his group. Schulten calls himself a mathematician by ability, who has a love for life sciences, but acknowledges that, back in the 1970s, it was tricky to apply mathematics to a new field like biology; there was much resistance and uncertainty. Schulten realized that to tackle this precarious task of introducing mathematics to biology he would need to work on solid ground and become thoroughly familiar with the experimental side. His time in Göttingen provided just that opportunity.
First, his experimentalist coworkers questioned Schulten's pure theoretical approach, which only made him improve his methods; there were also lots of good-natured jokes about him being a theoretician. Second, Schulten figured out how to apply a critical eye to experiment. “You need to know what are they doing, how are they doing it, who are the good people, and who are the not-so-good,” Schulten remarks. “So somehow you need to develop a working relationship.” Third, he learned about key directions in the field and heard about cutting-edge experiments while at the institute. In short, his time in Göttingen was not only productive in terms of research, but also because he made many contacts, with experimentalists in particular.
A Task Labeled Impossible
In 1980 Schulten's formative time at the Max Planck Institute came to an end, as permanent positions there were few. He took a job 245 miles away in Bavaria, as a professor in the physics department of the Technical University of Munich. He was still eight years away from revisiting photosynthesis in his own work, but as the decade wore on he had a front row seat to some cutting edge research that would eventually bring him back to this topic.

Hartmut Michel, who began a quest in 1978 to crystallize a membrane protein.
Southwest of the city center of Munich lies the town of Martinsried and in it is located the Max Planck Institute for Biochemistry. When Schulten moved to Munich in 1980 he began hearing about a fascinating project that was going on at the institute. A young biochemist there in Martinsried was trying to crystallize a membrane protein. Despite the claim in contemporary biochemistry textbooks that it was impossible to form crystals from this kind of protein, Hartmut Michel believed otherwise; an accidental observation had given him hope. Up to that point the proteins that had been crystallized were all solvated in water; since the surface of these proteins are electrically polar, they interact strongly with each other and are likely to form crystals. But membrane proteins live in lipid membranes, which are hydrophobic, and these proteins are largely nonpolar and so these membrane proteins don't interact strongly with each other.
In 1978 Hartmut Michel noticed by chance that the bacteriorhodopsin he was using in an experiment formed glass-like bodies when put in the freezer. At the time he was a post-doc in Dieter Oesterhelt's group in Würzburg, Germany, and Oesterhelt had discovered bacteriorhodopsin, an integral membrane protein, and proposed its function in the early 1970s. With the observation in the freezer, and with Oesterhelt's enthusiastic support, Michel spent the next year trying to form three-dimensional crystals of bacteriorhodopsin. While he was successful in finally obtaining the crystals, he discovered that they were not suitable for structural analysis in X-ray diffraction. Around this time Michel moved with Oesterhelt to Martinsried, where Oesterhelt became a director at the Max Planck Institute there.
But Hartmut Michel was not discouraged by his setbacks with bacteriorhodopsin. He decided to find more suitable membrane proteins and try and crystallize them instead. He chose to work with reaction center proteins from two purple bacteria, and also chlorophylls from spinach. He knew that these protein complexes were said to be part of crystalline arrays in their native environment. Using detergents, and techniques acquired from the bacteriorhodopsin work, in July 1981 Michel finally formed three-dimensional crystals of the reaction center protein of purple bacteria Rhodopseudomonas viridis. But the big test would be whether or not the reaction center crystals were of suitable quality for X-ray diffraction. In September of that year, working with Wolfram Bode, Michel found his crystals diffracted beautifully. Three years after Michel had accidentally observed crystalline-like membrane proteins in a freezer, he finally succeeded in producing high-quality crystals of a membrane protein, a task purported to be impossible. Michel points to 1981 as one of the best years of his life.
Once Hartmut Michel had his high-quality crystals, his next step was to find experts in X-ray crystallography in order to then elucidate the structure of the reaction center. Another group at the institute in Martinsried, led by Robert Huber, specialized in X-ray crystallography of proteins. In the spring of 1982 Michel gave a talk to Huber's group about his membrane protein crystallization work, seeking collaborators. Out of this seminar emerged a colleague for the project: Johann “Hans” Deisenhofer. Having joined Huber in 1971 as a PhD student, and then later converting that to a permanent position in the group, Deisenhofer did not hesitate for long to team up with Michel on the project, even though the reaction center was the largest protein at that time whose structure had yet to be determined and it was not clear that standard methods of structure determination would be suitable for the crystal.
Working at a rapid pace, and with input and support along the way from Robert Huber and Dieter Oesterhelt, the pair managed to solve the complete structure by 1985, helped by the following scientists: Kunio Miki, Otto Epp, Karl Weyer, and Heidi Gruenberg. By 1987 they had refined the structure at 2.3 Å resolution. Following their success, Hartmut Michel moved to Frankfurt in October 1987 to lead a group at the Max Planck Institute for Biophysics, and Hans Deisenhofer went to Dallas, Texas for a position at the Howard Hughes Medical Institute in March of 1988. In the fall of 1988 the two scientists, along with Robert Huber, would win the Nobel Prize in chemistry for determining the three-dimensional structure of the reaction center.
A Bonanza for a Computational Biophysicist
During his time in Munich, Schulten got to know Hartmut Michel and Hans Deisenhofer, and was always eager to hear about their progress on the reaction center over the course of the decade. In fact, Schulten soon realized that with the elucidation of the structure of a reaction center, many new calculations could be done that would have otherwise been impossible before the structure was solved. The reaction center solution had two major effects on Schulten's professional life. First, he returned to the field of photosynthesis, which had fascinated him from boyhood through graduate school. While he briefly touched on this topic in Göttingen, there he also learned about many of the major players at the forefront of photosynthesis research.
|
"THE REACTION CENTER SOLUTION HAD TWO MAJOR EFFECTS ON SCHULTEN'S PROFESSIONAL LIFE." |
Meanwhile, with the reaction center structure now in hand, in the late 1980s Schulten, working with Michel and Deisenhofer, began a series of molecular dynamics calculations on just a small section of the protein with his graduate student in Munich, Herbert Treutlein. They published a series of papers in 1988 on their molecular dynamics calculations. But one paper, written by Schulten, Deisenhofer and Michel, remained never to be published, a victim of fame. The referee report rejected the paper outright, claiming in this instance that one had to be super critical of high-profile scientists because otherwise the readership would easily believe everything the three famous scientists had written. Schulten is pretty sure this even happened before Michel and Deisenhofer received the Nobel Prize.
In the same year that he jump-started his return to photosynthesis by publishing computational papers that utilized the new reaction center structure, Schulten took a job at the University of Illinois at Urbana-Champaign. Soon after that, in 1989, Schulten founded the Theoretical Biophysics Group there at the Beckman Institute for Advanced Science and Technology, an interdisciplinary center at the university. Eventually the group at Beckman became known as the Theoretical and Computational Biophysics Group. It is through this group that Schulten and collaborators were able to start to piece together the many steps in photosynthesis and begin to understand it on a grand scale
Sir Anthony Leggett and Lord Rayleigh
One of the first projects Schulten embarked on at Beckman was an examination of electron transfer and its coupling to protein motion in the reaction center. This work culled from advances made by one of Schulten's colleagues, as well as one-hundred-year-old mathematics set forth by another famous physicist and Nobel Prize winner. During electron transfer in the reaction center, the electron is affected energetically by how the rest of the protein moves. The atoms in the protein vibrate due to thermal motion, and as the charges on the protein move around, the electrons exhibit slightly different quantum behavior. The reaction center protein has roughly 10,000 atoms, each vibrating in three directions, which means 30,000 vibrations. With a minimum of two parameters needed to describe the electron coupling, the result is 60,000 parameters to keep track of. To characterize this quantum system, Schulten and his graduate student Dong Xu had to dig deep.
A colleague of Schulten's in the physics department, and a next-door neighbor as well since 1988, is Sir Anthony Leggett. In 2003 Leggett would win the Nobel Prize in physics for his work explaining the theory behind superfluidity in helium-3. In 2004 Queen Elizabeth II knighted the London native for his discoveries. After his work in the 1970s that would earn him the Nobel Prize, one of the fields Leggett turned to was quantum mechanics under thermal conditions. Schulten recalls that among the many papers he published related to quantum mechanics, Leggett wrote a huge, comprehensive review article. “It was known in our physics department as the telephone book,” says Schulten, “because when it was a manuscript it was as fat as a telephone book when he sent it to the journal.”
Leggett's work on how quantum systems are affected by thermal disorder gave Dong Xu and Schulten the framework for describing how electrons in principle are coupled to the protein motion. They realized they did not explicitly need to know the 60,000 parameters, but instead could use certain functions that characterized how an electron is being affected by the vibrational motion. Additionally, Dong Xu did some detective work and hit on the mathematics derived by Lord Rayleigh in the late 1800s to describe how sound is generated; Rayleigh's equations worked out how the air, a totally disordered thermal bath, can still be imprinted with systematic coherent behavior to produce sound. “That kind of mathematics could actually be applied to the problem in the reaction center,” notes Schulten. “To understand the quantum motion of electrons, which is also coherent like sound, was the goal. The electron motions are actually being affected by the vibrations in the protein that act pretty much like the atoms in the air, statistically speaking.” Schulten is particularly proud of the elegant mathematical steps published in their papers on this topic.
After completing the electron transfer work, the mid-1990s arrived and brought an experimental collaborator from Schulten's past back into the picture. Little did Schulten realize that one inebriated evening would lead to one of the most highly cited papers in his whole career.
Intrepid Spirit Needed
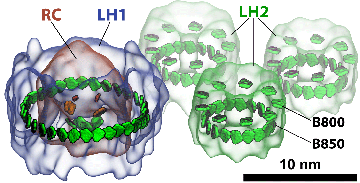
Once the reaction center had been solved in the mid-1980s, its structure promised that the physics behind it could now be resolved on firm grounds. But the reaction center was not the only protein that existed in the photosynthetic apparatus of purple bacteria. Two other types of proteins were important for absorbing light: light-harvesting complex 1 (LH1) and light-harvesting complex 2 (LH2). It was known that these complexes were made of pigments that absorb photons and then transfer that absorbed energy to the reaction center. Hence the term “light harvesting” can be thought of as the process of capturing light and then transferring that energy. Structural information about LH2 was minimal in the early 1990s, which made it impossible to firmly deduce the physics behind how LH2 functioned. But all this was about to change, as two groups would race to publish their LH2 structures first.
Xiche Hu,circa 1995, who worked on solution of light-harvesting proteins.
Sometime around 1994 Hartmut Michel, now in Frankfurt, was visiting the Schulten group at Beckman, invited by Schulten to give a lecture. At a party Schulten hosted during the visit, Schulten and Michel got a bit inebriated and thus a bit emboldened, and decided to solve the structure of a light-harvesting complex Michel had been working on, even though a key piece of information was missing. Up to that point various light-harvesting proteins had been crystallized, but none explicitly solved. The two scientists made a deal: Michel's group would hand over their diffraction data, and Schulten's group would have a certain amount of time to solve the protein structure with Michel's diffraction data.
When Hartmut Michel's group began work on crystallizing LH2, another researcher at the University of Glasgow began similar determination of the structure of LH2 as well, but for a different bacterium. The British scientist, Richard Cogdell, worked on structure determination for the species Rhodopseudomonas acidophila. On the other hand, Hartmut Michel had chosen to work on LH2 of the bacterium Rhodospirillum molischianum, a decision that would have profound consequences.
Since this was such a tricky problem to solve, Schulten put a big group of people on it: three graduate students and a new post-doc, chemist Xiche Hu. Over the course of several months, as the intricacy and complexity of the problem grew and grew, everyone would drop out of the project except Xiche Hu, the others daunted by what was known as the phase problem.
When diffraction data are generated from a crystal, only two pieces of data can be extracted from the experiment. The third piece of data, missing but necessary for elucidating the structure, is the phase angles of the reflections. One way to solve this is to grow the crystal with heavy metals that are bound to particular places in the protein and through geometrical constructions determine the phase. But the protein Rs. molischianum was so modular that the experimentalists in Michel's group could not co-crystallize with heavy metals successfully. So it fell to Xiche Hu and Klaus Schulten alone to come up with a way to conquer the phase problem.
For Schulten and Hu, two blows would soon befall them as Hu worked diligently on the phase problem. First, they learned that Richard Cogdell and his collaborators succeeded in solving the structure of Rps. acidophila, and would soon publish in Nature. The light-harvesting protein that Hu and Schulten were working on turned out to be the more difficult one to solve. And second, since the allotted months had passed and Hu did not have a structure, the problem reverted back to Hartmut Michel's group. Although this relinquishment was a blow to the scientists in Urbana, Xiche Hu was philosophical about it. “In some sense they had the right to do it,” Hu remarks, “because in principle they didn't need us to solve the structure.”
Although Xiche Hu had to teach himself the entire field of crystallography to work on the light-harvesting complex, he had learned quite a few things during his limited time working with the diffraction data. So when Hartmut Michel was unable to solve the LH2 structure, he approached Schulten to rejoin the project. Xiche Hu was ready to revisit the challenge. “Xiche Hu came every morning to my office,” recalls Schulten, “and put his arm around my shoulder and said, 'Klaus, we are going to make it.' So he, not the postdoctoral advisor, was the confidence generator.”
To conquer the problem, Hu and Schulten first generated a structure that they hoped was similar to the real structure, which they called a “search model,” and which they based on the Rps. acidophila structure that had already been determined by Cogdell's group. The experimentalists in Michel's group were experts in reading diffraction data, and surmised that the structure of the light-harvesting complex was a ring made of eight identical subunits, which effectively reduced the problem to one-eighth of its original complexity. So Hu and Schulten only needed to solve the structure for one subunit in the protein, and they could utilize Cogdell's structure as a guide. In essence Hu and Schulten used a computational step to generate search models that were homologous to the unknown structure so they could determine the phases. Schulten likens the procedure to iterating with Newton's Method: if the answer diverges, one can clearly see the initial guess was far off and so one starts over with a different guess. Hu says that what finally solved the structure was exploiting the symmetry aspects of the protein. The structure they computed is shown at left.
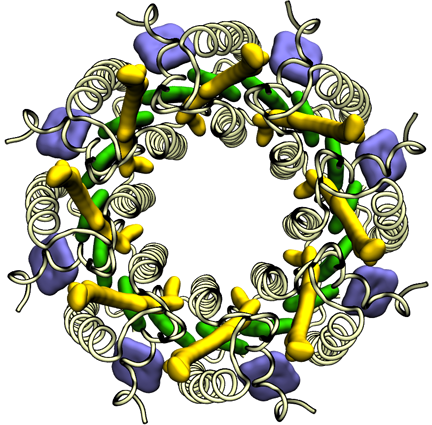
The light-harvesting complex solved by Klaus Schulten and Xiche Hu in 1995.
Schulten calls the moment when he saw the structure of the light-harvesting protein one of the peak highlights of his entire career. He realized the significance of this work immediately. His reaction, however, was not quite the same as his competitor, Richard Cogdell's. “So Cogdell tells me, every time I see him: Oh Klaus, I'll never forget when I saw the structure at first, I was just weeping,” recounts Schulten. Although the two teams were racing to solve their respective structure first, the team headed by Michel and Schulten published about a year later than Cogdell. But this publication, in May of 1996 in the journal Structure, remains one of Schulten's most highly cited papers, with 821 citations as of July 2012.
Confrontation in Spain
Although the project culminated in a good outcome, Schulten confesses he had his doubts all along the way: was the ring really eight-sided, did they have the correct symmetry group, would a useful search model ever surface? And these doubts were only exacerbated by a confrontation Schulten had at a conference, before the structure of their light-harvesting protein was successfully solved.Around 1995 Schulten went to Spain for a meeting, and gave a talk about the computational methodology Xiche Hu and Schulten were using to approach the problem of solving the structure. Basically Schulten was talking about the role the theorist could play in X-ray crystallography, a field usually reserved for experimentalists. In the audience was crystallographer Robert Huber, who had won the Nobel Prize in 1988 for his role in determining the structure of the reaction center protein. “Huber shot up afterwards, after my lecture,” recounts Schulten, “and said this is the greatest rubbish he ever heard, totally undoable and really stupid.”
Schulten thought this was way too aggressive, and he was not the only one. “There was a coffee break,” continues Schulten, “and after the coffee break, several colleagues stood up and they said: We have to comment on this, because the exchange you heard is making a very bad example for our young people.” These scientists basically conveyed that to not try new approaches, like the one Schulten was advocating, is exactly against the fabric of science. They were not dissuaded by the fact that Huber was a Nobel laureate.
Huber was privy to the whole exchange that followed the coffee break. He eventually stood up and apologized. “He said he would only be too happy if theory would replace experiment,” Schulten recalls. But Schulten recognized that Huber did not actually mean it. This whole incident was only one of many factors that contributed to Schulten's doubts about the success of the project to determine the light-harvesting protein's structure. In the end, however, his doubts were put to rest. And the structure determination was only the beginning of one of the most impressive projects ever achieved in his group.
From Structural Biology to Quantum Physics
With the structure of a light-harvesting complex under his belt, Xiche Hu set out to use the same technique he had honed in his LH2 work to predict the structure of a light-harvesting complex 1 for a different purple bacterium. Around this time two European graduate students joined the group at Beckman and started to elucidate the physics behind light harvesting, work made possible by the newly unveiled structures of LH1 and LH2. The work at Beckman would shift from structural biology to quantum biology.While he was studying for his diploma at the University of Frankfurt in Germany in the early 1990s, Thorsten Ritz heard Klaus Schulten give a seminar there about a possible mechanism for the magnetic compass in birds. Captivated by the talk, since it pointed out one of the ways in which quantum mechanics plays a role in biology, Ritz made sure to talk to Schulten while he was briefly doing research at the Center for Complex Systems Research in Urbana. Ritz decided to do dissertation research with Schulten in Urbana while getting his PhD from the University of Ulm. As Schulten did not have a candidate molecule yet for the avian magnetic compass, Ritz began his studies on photosynthesis instead.
|
"THE EXCHANGE YOU HEARD IS MAKING A VERY BAD EXAMPLE FOR OUR YOUNG PEOPLE." Klaus Schulten |
In a similar vein, Ana Damjanović was an undergraduate student in physics at Belgrade University when she met Schulten at a conference in Europe. Impressed by his research, she went to the University of Illinois at Urbana-Champaign specifically to get her PhD in Schulten's group. Shortly after her arrival, she would team up with Ritz and begin work on the quantum physics of photosynthesis.
One of the first things the trio would immediately uncover was how quantum coherence assists light harvesting. In the two light-harvesting proteins, LH1 and LH2, chlorophyll-like molecules (technically called bacteriochlorophylls in purple bacteria) are packed closely together in a ring shape, as seen in the picture of LH2 for example. To transfer the energy harvested by the chlorophylls, the individual chlorophylls team up and transfer the excitation not randomly but in a pool. They share their excitation in a very ordered, or so to speak, “coherent” way; it is as though they are humming one tune together as opposed to each playing unique parts in an orchestra. With quantum coherence, the system of pigments could reach very far and fast to transfer the excitation.
In the 1940s Arnold and Oppenheimer had wondered why so many chlorophylls were necessary in photosynthesis and exactly how they all worked together. In the late 1990s, Schulten together with Hu, Ritz and Damjanović elucidated the physics underlying the structures of the light-harvesting proteins, namely that the arrangement of a group of tightly-interacting chlorophylls in fact act together to make themselves more efficient through quantum coherence.
Return to Polyenes
The light-harvesting pigments are not only made of chlorophyll-like molecules that absorb sunlight. They also consist of carotenoids, which are pigments that also absorb light, usually in the blue range, and are popularly known for their nutritional value in foods such as tomatoes and sweet potatoes. The other main highlight of the collaboration between Damjanović and Ritz was clarification of the role that carotenoids play in light harvesting. Basically they found that the carotenoids have to use tricks in order to transfer the excitation to the chlorophylls.
In this study of carotenoids, a trip to Japan for an unrelated reason rekindled Schulten's interest in his Harvard work from the early 1970s on polyenes. Carotenoids are related to polyenes, as both share an underlying structure of conjugated double bonds, and both polyenes and carotenoids have low-lying, optically forbidden states. While Schulten was in Japan in the late 1990s for a conference on visual receptors, he met Yasushi Koyama, an experimentalist who had studied the forbidden states in polyenes. Koyama was keen to collaborate with Schulten and, in order to get to know him better, asked Schulten if he would like to take a drive to an earthquake museum. The Kobe earthquake rocked Japan in 1995, and a memorial museum commemorated the devastation.
During the trip to the earthquake museum, Koyama revealed he was aware of the work Schulten and Karplus did in the early 1970s to prove that polyenes had optically forbidden states, and that he was very eager to team up with Schulten's group for a paper. Schulten and Koyama, together with the team of Ritz and Damjanović, elaborated on the mechanism that made carotenoids sometimes couple efficiently to chlorophylls to transfer excitation when the coupling was expected to be poor. They suggested in a 2000 paper that some photosynthetic systems have a type of symmetry breaking in their carotenoids that augments the excitation transfer efficiency.

Klaus Schulten, Ana Damjanović and Thorsten Ritz, who elucidated the quantum physics of photosynthesis, circa 1998 or 1999.
This period of time in Schulten's group, from about 1995 to 2000, still stands out as one of the most impressive eras of achievement in Schulten's mind. It started with Xiche Hu and his relentless efforts to solve the structure of LH2, and then ended with Thorsten Ritz and Ana Damjanović. The pair basically sorted out the key physical characteristics that nature had designed to effectively absorb sunlight, to keep it for a short while and transfer it effectively to other pigment subsystems in the so-called photosynthetic light harvesting system.
When asked why the team of Hu, Ritz, and Damjanović was able to unravel the physics behind light-harvesting in such a compact amount of time, Damjanović points to three reasons: the team worked extremely hard, she and Thorsten Ritz helped each other cooperatively, and when stuck, Damjanović took full advantage of the many resources available. “There was usually someone around who knew how to use the software or programs that I needed to use,” Damjanović clarifies, “If I got stuck somewhere, I would go and ask them for help.” This way Damjanović avoided gridlock in the research, by promptly seeking help from scientists at the University of Illinois, and sometimes even researchers at other institutions.
Schulten also weighed in on why the team of Ritz and Damjanović made remarkable achievements. Xiche Hu's work on the light-harvesting protein was the first time in Schulten's group that someone had worked on structural biology. “This structure determination was very new for us; but particularly relative to the structural biology, we were really excellent quantum biologists,” notes Schulten. This second hat that the team wore, of quantum physicists, was a natural one for Schulten's group to embrace since they could draw on years of experience, and this resulted in explanation of the quantum physics behind light harvesting in photosynthesis in the near-record time of about three years.
From Molecule to Cellular Compartment
With the advent of the twenty-first century the team at Beckman was engaged in a whole host of photosynthesis work. Thorsten Ritz and Ana Damjanović were finishing up their explorations of light harvesting, and others in the group were expanding from purple bacteria to cyanobacteria and plants. In the middle of this first decade of the new millennium a chance meeting at dinner would bring Schulten closer to his dream of explaining the workings of a living cell from its constituent molecules. He would now go from molecule to organelle.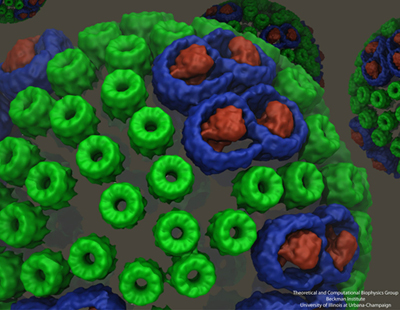
The chromatophore, a compact cellular compartment. LH1 proteins in blue surround the reaction center in red; LH2 are the green rings.
When Schulten moved to Illinois in 1988 he eventually met two scientists there at the university who also worked on photosynthesis: Antony Crofts and Colin Wraight, both from the School of Molecular and Cellular Biology. Schulten even published a photosynthesis paper in 1999 with Crofts. When Colin Wraight invited Schulten to a dinner in honor of a visitor from Sheffield, Schulten and the guest from England struck up a conversation about their respective work in photosynthesis. Neil Hunter, an experimentalist, was at that point in time imaging a structure called a chromatophore that was central to photosynthetic function in purple bacteria. Always on the lookout for collaborators, Schulten and Hunter decided to join forces to model the chromatophore.
Melih Sener, with a PhD in theoretical high-energy physics, started working on modeling the chromatophore in 2005. While high-energy physics and biophysics may seem like disparate fields, Sener had a clear objective for making the switch. “It's a little bit like the history of Don Quixote,” Sener explains. “He read knightly stories and attacked windmills. And I read the stories of all the heroes of nineteenth- and twentieth-century physics and when I realized that most windmills were already overtaken in high-energy physics, I decided that the most interesting problems are then in biological physics.”
A chromatophore is a complete cellular compartment that's part of a bacterial photosynthetic membrane. “It's essentially a machine that captures sunlight and converts it to chemically stored energy in the form of ATP, and it does so by a number of cooperating proteins,” Sener says. Similar to an organelle in eukaryotes, in bacteria this chromatophore is termed a pseudo-organelle. The chromatophore that Sener began working on is found in the purple bacterium Rhodobacter sphaeroides, contains several hundred proteins, and has a spherical shape that is in vesicle format, although not all chromatophores are spherical. One of the main tasks that Sener faced in the modeling project he light-heartedly compares to the forensics of road kill.
“The way these things are imaged is that you take the vesicle, and then squeeze it flat on a mica surface. Then you can scan it with an atomic force microscope,” explains Sener. With projection algorithms borrowed from geography, Sener deployed computing tools on a flattened patch to reconstruct what it might have looked like when spherical. In fact, Sener utilized experimental data from many sources, not only atomic force microscopy, to reconstruct the chromatophore. He cites as a major challenge the accrual and utilization of decades of experimental data. He culled from X-ray crystallography, NMR, electron microscopy, spectroscopic data, and, of course, the atomic force microscopy images from Neil Hunter's lab. Creation of the model, as shown above, is still an ongoing project for Sener in Schulten's group. For example, missing components need to be filled in, but experimental collaborators are working on some of these issues. To the best of his knowledge, however, this is most likely the first attempt to combine so much data into one structural, organelle-like model.
But this generation of the three-dimensional blueprint of the chromatophore was not the only goal. Sener removed the cap of the forensic scientist and replaced it with the cap of the physicist. His objective: use the tools of quantum physics to model light harvesting for the entire chromatophore. This stage, as well, presented multi-layered challenges to Schulten and Sener. One of them was the separation of scales, time scales as well as length scales. They wanted to describe the many processes involved in converting absorbed energy to ATP, but each step involved different time scales. For example, the first processes of light absorption and excitation transfer are femtoseconds to picoseconds, while the later processes of chemical transformations climb toward microseconds and milliseconds. Length scales also varied from describing electronic states distributed over a single molecule to as large as over the entire vesicle, 50 to 70 nanometers. “So naturally,” summarizes Sener, “you can't use one formulation, one language, one computational framework” when working with disparate scales.
The overall goal of this second stage of the chromatophore project is to compute the rate of ATP production as a function of incoming light intensity. “This required us to look at the chromatophore as a complete machine, where multiple processes communicate with one another,” Sener remarks. “And that's probably going to keep us busy for some time.”
Membrane Curvature
While Sener and his many experimental collaborators were keeping busy modeling the chromatophore, Schulten put other group members on the project of self-assembly of photosynthetic membranes. As shown in the graphic above, the chromatophore consists of light-harvesting proteins (LH2 in green, and LH1 in blue with the reaction center in red) among its many components. Schulten had the idea that these light-harvesting proteins, which have a functional role within the chromatophore, may also help to shape the structure itself as the proteins of the chromatophore aggregate to form membrane indentations.
With papers published in 2008 and 2009, molecular dynamics calculations on three different bacteria showed that aggregates of LH2 complexes do seem to induce curvature, which is consistent with the curved chromatophore membrane structures seen in certain kinds or purple bacteria. These various calculations encompassed between 880,000 and 1.7 million atoms. The scientists who worked on the curvature properties in these two publications include Danielle Chandler and Jen Hsin.
While Hsin and Chandler were pursuing the study of membrane curvature in 2008, a string of lucky coincidences occurred that would enable Hsin to elaborate what is known as the core complex in atomic detail. A burgeoning method in Schulten's group and some new experimental data materialized at exactly the right moment to further knowledge of the core complex.
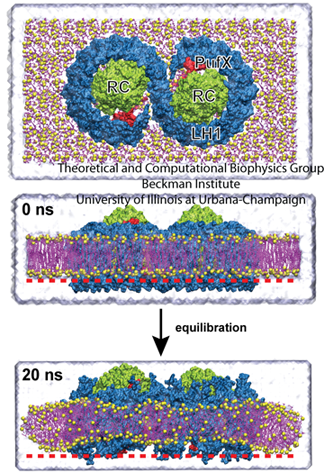
The top image shows the core complex, while the bottom image shows the degree of bending seen in simulations.
Klaus Schulten's group has many areas of study, including software development as well as various fields of biophysics, such as magnetoreception, bionanotechnology, and of course, photosynthesis. But in 2005 he agreed to collaborate on a project with an experimentalist that promised a fascinating molecular machine to work with, and that would be a great test for the methodology and techniques his group has developed over the years to examine massive systems. What he and his students needed to do was combine data from X-ray crystallography with lower resolution data from the electron microscope, and the system in question was the cell's protein making factory: the ribosome. Essentially, to use an analogy, consider trying to understand a bird in flight. The X-ray crystallography data captures a crisp picture of a bird standing still on a wire, with the position of every claw, feather, and eye well known. But the electron microscopy image of a flying bird is more fuzzy, although captures the bird in the course of its function. What Schulten needed to do was apply all the crisp details of the motionless bird to the image of the bird in flight, therefore making it possible to explain flight, or the bird's function.
All the elements to coax the X-ray data into the electron microscopy image of the ribosome were at hand in Schulten's group; in fact his career was ready for it by 2006. He had in-house software, VMD and NAMD, which group members could exploit and alter, he had a mixture of software developers and research scientists who could offer support and expertise, he had a thirty-year history of working with experimentalists, his group at the Beckman Institute was a well-oiled machine after 20+ years, and he had energetic and talented graduate students who worked tirelessly on the ribosome. So, in a matter of about two years, his group had developed a method they introduced to the world in 2008, called Molecular Dynamics Flexible Fitting (MDFF). A detailed account of its creation and subsequent numerous applications can be found in this history of MDFF.
Jen Hsin had been studying the core complex, which, as seen in the image, is comprised of two reaction centers in green with light-harvesting complex I (LH1) wrapped around it in blue. With help from fellow graduate student James Gumbart, Hsin built a model of the core complex, which is considered a dimer since it has two distinct but similar regions. She used bits and pieces of proteins to make an atomic-level structure of the dimer. And when she ran simulations of the dimer in a patch of membrane, the system wouldn't stay flat, an effect seen in the bottom frame of the image. “We were puzzled by this result, as such geometry was unusual for integral membrane proteins,” recalls Hsin. “Until our collaborators informed us that they had just obtained a low-resolution electron microscopy map of the same protein complex showing that it is indeed bent, and even more drastically than we saw in the simulation.” The experimentalist who provided the electron microscopy data was Neil Hunter.
To further their understanding about how the large degree of bending they were seeing, now on two fronts, would affect membrane geometry, the task at hand required merging the atomistic models and simulations of Hsin's with the low-resolution map from Neil Hunter. At this time, the method Molecular Dynamics Flexible Fitting had just been developed in the Schulten group, which was exactly the method needed to merge atomic-level structures into less-resolved electron microscopy images. Coincidentally, all the pieces of the puzzle came together so perfectly to further the study of membrane curvature with MDFF. Hsin explains that the MDFF work, published in 2009, showed that “higher order structures of large, transmembrane protein complexes can force their membrane environment to adopt to their geometries.”
Membrane curvature research is still ongoing. Currently Danielle Chandler is continuing its study, with massive calculations that are pushing the limits of the simulation and visualization software produced in Schulten's group, namely NAMD and VMD. Her simulations encompass 20 million atoms, probably one of the largest molecular dynamics calculations in the world on a biological system to date. Membrane curvature is not the only physical property of study. “We're also interested in things like protein-lipid interactions, lipid mobility, etc.,” Chandler says.
The purple bacterium in question is Rhodospirillum photometricum, chosen because of the availability of atomic force microscopy data on the structure. But all this data presents major challenges for the researcher. One of the steps of this molecular dynamics simulation involves gathering coordinates for 20 million atoms, but the software VMD can only tackle a few million atoms. “John Stone, the developer of VMD in Schulten's group, had to invent a new file format to handle it,” continues Chandler. “The new format is a binary file format–so you can't open it in a text editor to look at it, but it can handle an arbitrarily large amount of data.” While the coordinates of Rsp. photometricum take up approximately 1.1 gigabytes, a trajectory file from a molecular dynamics run is about 4 terabytes large, and has to be stored on external hard drives. This calculation is already running on the Blue Waters supercomputer located at the University of Illinois at the National Center for Supercomputing Applications (NCSA). Completion of these massive computations will only bring the Schulten group closer to its goal of predicting the behavior of entire life forms from just their constituent atoms.
The Role of Quantum Coherence, Revisited
The final stage in this history of photosynthesis ends with a look at what the group at Beckman is doing most recently on quantum coherence in photosynthetic purple bacteria. In 1999 Schulten and his graduate students Ana Damjanović and Thorsten Ritz investigated how quantum coherence manifests in excitation transfer in the purple bacteria; at the close of this first decade of the new millennium Schulten returned to this topic, but in a much more comprehensive and elegant manner.
In 2009 graduate student Johan Strümpfer joined Schulten's group and faced a great challenge: find and implement a theory to explain excitation transfer in the pigments of purple bacteria that is free of all the assumptions that undergird the theory describing excitation transfer up to that point.
Light-harvesting proteins. Studies were done on how excitation is transferred from one ring to another, as well as within a single ring.
Coherence, which is a quantum phenomenon mentioned above and first described in the work of Xiche Hu, Thorsten Ritz and Ana Damjanović, means that two things, like molecules in a pigment ring, are correlated. “So coherence is like taking two things and making them almost one,” Strümpfer explains, “where one directly affects the other one instantaneously. There's no time delay, there's no exchange of anything, it's just an instantaneous thing.” For example, chlorophylls in a ring of a light-harvesting protein are involved in sharing excitation. “When chlorophylls share the excitation between them,” Schulten further clarifies, “it's not like the excitation hops from one chlorophyll to the next and then totally forgets where it comes from. Rather it remembers where it comes from.” This is one manifestation of coherence in photosynthesis.
One of the things Strümpfer had to account for was the thermal environment. Very often physicists like to do their calculations at absolute zero to reduce the complexity of a difficult problem. But Strümpfer did not have that luxury with living systems. “You have to work at room temperature, or the temperatures that these organisms experience,” Strümpfer explains. “And consider everything in place there: the membrane, the water, and the proteins surrounding everything. You know that those components are fluctuating with thermal noise basically.”
While taking into account all the molecules in a protein, and the lipid membrane it is attached to, and the water that surrounds it, may seem like a daunting task, Strümpfer and Schulten used clever mathematics to circumvent the problem of including those millions of atoms in the surrounding environment. The scientists again turned to Sir Anthony Leggett, who has studied quantum mechanics in a thermal environment. The elements of the surroundings, like water molecules or the lipids of a membrane, vibrate with a certain set of frequencies; Strümpfer used a distribution of frequencies that reflected the surrounding pieces and coupled the pigments he was studying to those frequencies. Strümpfer and Schulten basically took the ideas of Leggett and adapted them to their particular problem.
While Strümpfer had successfully unearthed a theory to explicate excitation transfer, implementation presented immediate challenges. Not only was the new theory hard to understand, writing code that ran fast enough was a hurdle. Strümpfer discovered that he had to educate himself very quickly about computer hardware and the nuances of writing efficient C++ code. This calculation required huge amounts of RAM, 200 gigabytes, and only one computer in the group met those conditions; but getting his code to take advantage of the huge memory was not straightforward. “I had to scramble and ask people all over the place how to do this. I needed to get it faster. I couldn't do the calculation unless I could get it done quickly.” Strümpfer eventually got his code working.
The graphic above shows a small segment of a photosynthetic apparatus, which includes a reaction center that is surrounded by an LH1 ring, and close by are three LH2 rings. The LH1 ring basically acts as a system of antennae, feeding excitation to the reaction center. But in the dark habitats that these purple bacteria live in–for example, in the bottom of ponds–more feeder systems evolved, namely LH2s, to channel additional excitation energy to the LH1 and then to the reaction center. In the work Strümpfer and Schulten did on excitation transfer, they looked at transfer in two distinct forms: how excitation travels within a ring, and how excitation travels from one ring to another. And they found some surprising results.
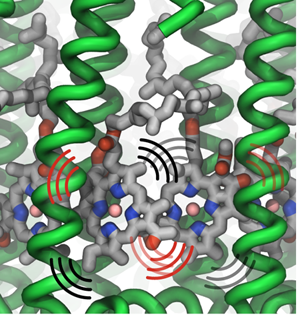
Thermal vibrations in LH2 proteins.
In considering how excitation moves within a ring, say within an LH1 ring or within one LH2 ring, Strümpfer looked at exciting just one pigment molecule individually in a ring and then observed how it settled. “Suppose excitation starts at one place,” spells out Strümpfer, “and then while this is still excited, another one also becomes excited. You have both of them excited at the same time, and then if it's very strongly coupled, then they're coherently excited. So then they act as one unit now, instead of being two separate things.” Strümpfer goes on to note that the description he and Schulten like for this inter-ring coherent behavior is to consider it as waves sloshing back and forth, or running around in the ring. “This is something that I struggled with immensely, actually,” admits Strümpfer, “because we don't have things that behave like this in our reality, if you can imagine.”
Strümpfer and Schulten found that within a ring complex, coherence plays an important role; but the hopping from one ring to another, for example from one LH2 to a different LH2, is much easier to visualize and compute. Excitation hops from one ring to another, and its mathematics is given by probabilities, but the excitation is never in both rings at the same time. “So that makes it easier, first, to imagine, and then second, to actually model physically,” remarks Strümpfer.
One of the major questions people have asked Schulten and Strümpfer about this work is how does quantum coherence then play a role in light harvesting. And Strümpfer calls the answer one of the most surprising findings to come out of their comprehensive study. “The effect is that having quantum coherence within one ring dramatically improves the rate at which excitation can jump from one ring to the next ring,” he summarizes.
Schulten is proud of his group's efforts on coherence, which started in the late 1990s and continues on today with the work of Johan Strümpfer. But this current work on coherence draws from some 30-plus years of Schulten and collaborators laboring to understand the story of how nature has fashioned photosynthesis to make it an efficient and elegant process. And Schulten's objective still remains to uncover the many steps of photosynthesis and clearly explain the findings to the research community. “There was a lot of work done, methodological developments and theory and so on, but the goal was to explain how nature effectively harvests sunlight,” summarizes Schulten on his last 35 years. “It's maybe still not complete, but I figured it out to a large degree.” And without a doubt, for what remains to be done, Schulten's group will be at the forefront in the years to come.
Epilogue
After forty-plus years of studying photosynthesis, Klaus Schulten has pieced together the intervening processes that occur between the absorption of sunlight and the generation of ATP. He feels he has finally garnered an understanding to suit himself, about the full mechanism of the photosynthetic membrane. With solution of the entire structure of the chromatophore at hand, Schulten was at a point in his career where he could illustrate the function of this autonomous machine, which harbors many processes that interlock together to make photosynthesis work. He had also demystified the role of each type of protein that comprises the chromatophore. At this juncture in time (2014), he harkened back to his long-cherished goal of explaining a whole cell, and realized he could by now reach this goal, by at least explicating a living system at the organelle level. He could show the function of chromatophore and how its proteins worked together like a Swiss watch to convert sunlight into ATP. But what he wanted to do scientifically he could only do with a movie.
This is no Hollywood creation, however, with its myriad special effects and artists' renditions. Schulten had control of all 100 million atoms that make up the chromatophore, and each interlocking process illustrated is based on sound research conducted over decades by many scientists, including various members of Schulten's group. The movie starts with light harvesting and quantum coherence, proceeds to electron and proton transfer, and ends up with synthesis of ATP. The proteins critical to this array of processes are the reaction center, LH1 and LH2, the bc1 complex, and finally ATP synthase. Within the context of the chromatophore, the movie accounts for all the interacting parts for an entire, multi-faceted description of a full biological machine. What is illustrated is the interdigitation of processes going on, not just one process or one protein, as is often how scientists have to study living systems.
This movie is a culmination of a life's career in the field of photosynthesis, based on over forty years of work. It also illustrates a driving principle for Klaus Schulten, the desire to understand biological organization, or how multiple proteins form societies, or associations and collaborations, and thereby make a cell become "alive." The movie indeed looks at one such society with over one hundred proteins enclosed in a membrane, encompassing roughly 100 million atoms. Get ready to see how this biological machine functions, based on decades of work at the level of atoms and molecules.
Photosynthesis Movie
The Photosynthetic Membrane of Purple Bacteria: A Clockwork of Proteins and Processes from TCBG on Vimeo.
Other Related Articles
If you enjoyed this article, you might be interested in these other histories:History of Magnetoreception
History of Polyenes
History of Membrane Proteins
History of Bionanotechnology
History of NAMD
History of VMD
History of MDFF (Molecular Dynamics Flexible Fitting)
Comments?
For comments or inquiries, Lisa Pollack can be reached at the following: lpollack AT ks.uiuc.eduCitation
To cite this article, please use the following:Pollack, Lisa. "Unraveling Photosynthesis Step by Step: Four Decades of Research in Theoretical and Computational Biophysics." www.ks.uiuc.edu. 1 September 2012, from http://www.ks.uiuc.edu/History/photosynthesis/
References
Stephen Jay Gould. Planet of the Bacteria. Washington Post Horizon, 119:344, 1996.
Martin Karplus. Spinach on the Ceiling: A Theoretical Chemist's Return to Biology. The Annual Review of Biophysics and Biomolecular Structure, 35:1-47, 2006.
Klaus Schulten and Martin Karplus. On the origin of a low-lying forbidden transition in polyenes and related molecules. Chemical Physics Letters, 14:305-309, 1972.
Klaus Schulten, H. Staerk, Albert Weller, Hans-Joachim Werner, and B. Nickel. Magnetic field dependence of the geminate recombination of radical ion pairs in polar solvents. Zeitschrift für Physikalische Chemie, NF101:371-390, 1976.
Hans-Joachim Werner, Zan Schulten, and Klaus Schulten. Theory of the magnetic field modulated geminate recombination of radical ion pairs in polar solvents: Application to the pyrene-N,N-dimethylaniline system. Journal of Chemical Physics, 67:646-663, 1977.
Zan Schulten and Klaus Schulten. The generation, diffusion, spin motion, and recombination of radical pairs in solution in the nanosecond time domain. Journal of Chemical Physics, 66:4616-4634, 1977.
Hans-Joachim Werner, Klaus Schulten, and Albert Weller. Electron transfer and spin exchange contributing to the magnetic field dependence of the primary photochemical reaction of bacterial photosynthesis. Biochimica et Biophysica Acta, 502:255-268, 1978.
Web of Stories. “Manfred Eigen, 108, The winter seminars.” Accessed April 12, 2012. http://www.webofstories.com/play/52329?o=MS
Web of Stories. “Manfred Eigen, 109, Why the winter seminars are unique.” Accessed April 12, 2012. http://www.webofstories.com/play/52330
Theodor Förster. Zwischenmolekulare Energiewanderung und Fluoreszenz. Ann. Phys. (Leipzig). 2:55-75, 1948.
J. R. Oppenheimer. Internal Conversion in Photosynthesis. Phys. Rev., 60:158, 1941.
William Arnold and J. R. Oppenheimer. Internal conversions in the photosynthetic mechanism of blue-green algae. J. Gen. Physiol., 33:423-435, 1950.
William A. Arnold. Personal Perspective: Experiments. Photosynthesis Research, 27:73-82, 1991.
Nobelprize.org. “Hartmut Michel - Autobiography.” Accessed April 13, 2012. http://www.nobelprize.org/nobel_prizes/chemistry/laureates/1988/michel-autobio.html
Nobelprize.org. “Johann Deisenhofer - Autobiography.” Accessed April 12, 2012, http://www.nobelprize.org/nobel_prizes/chemistry/ laureates/1988/deisenhofer-autobio.html
Nobelprize.org. “Video Player: Interview with Johann Deisenhofer,” Accessed April 12, 2012, http://www.nobelprize.org/mediaplayer/index.php?id=893&view=1
Nobelprize.org. “Video Player: Interview with Hartmut Michel,” Accessed April 13, 2012, http://www.nobelprize.org/mediaplayer/index.php?id=413&view=1
Johann Deisenhofer and Hartmut Michel. The photosynthetic reaction centre from the purple bacterium Rhodopseudomonas viridis (Nobel Lecture). Angew. Chem. Int. Engl., 28:829-847, 1989.
Johann Deisenhofer and Hartmut Michel. The photosynthetic reaction centre from the purple bacterium Rhodopseudomonas viridis. Science, 245:1463-1473, 1989.
Herbert Treutlein, Christoph Niedermeier, Klaus Schulten, J. Deisenhofer, H. Michel, Axel Brünger, and Martin Karplus. Molecular dynamics simulation of the primary processes in the Photosynthetic reaction center of Rhodopseudomonas viridis. In A. Pullman et al., editors, Transport Through Membranes: Carriers, Channels and Pumps, pp. 513-525. Kluwer Academic Publishers, Dordrecht, 1988.
Herbert Treutlein, Klaus Schulten, Christoph Niedermeier, J. Deisenhofer, H. Michel, and D. Devault. Electrostatic control of electron transfer in the photosynthetic reaction center of Rhodopseudomonas viridis. In J. Breton and A. Verméglio, editors, The Photosynthetic Bacterial Reaction Center: Structure and Dynamics, volume 149 of NATO Science Series A: Life Sciences, pp. 369-377. Plenum, New York, 1988.
Dong Xu and Klaus Schulten. Coupling of protein motion to electron transfer in a photosynthetic reaction center: Investigating the low temperature behaviour in the framework of the spin-boson model. Chemical Physics, 182:91-117, 1994.
Juergen Koepke, Xiche Hu, Cornelia Muenke, Klaus Schulten, and Hartmut Michel. The crystal structure of the light harvesting complex II (B800-850) from Rhodospirillum molischianum. Structure, 4:581-597, 1996.
Ana Damjanović, Thorsten Ritz, and Klaus Schulten. Energy transfer between carotenoids and bacteriochlorophylls in a light harvesting protein. Physical Review E, 59:3293-3311, 1999.
Thorsten Ritz, Ana Damjanović, Klaus Schulten, Jian-Ping Zhang, and Yasushi Koyama. Efficient light harvesting through carotenoids. Photosynthesis Research, 66:125-144, 2000.
Melih K. Sener, John D. Olsen, C. Neil Hunter, and Klaus Schulten. Atomic level structural and functional model of a bacterial photosynthetic membrane vesicle. Proceedings of the National Academy of Sciences, USA, 104:15723-15728, 2007.
Danielle Chandler, Jen Hsin, Christopher B. Harrison, James Gumbart, and Klaus Schulten. Intrinsic curvature properties of photosynthetic proteins in chromatophores. Biophysical Journal, 95:2822-2836, 2008.
Jen Hsin, Danielle E. Chandler, James Gumbart, Christopher B. Harrison, Melih Sener, Johan Strümpfer, and Klaus Schulten. Self-assembly of photosynthetic membranes. ChemPhysChem, 11:1154-1159, 2010.
Jen Hsin, James Gumbart, Leonardo G. Trabuco, Elizabeth Villa, Pu Qian, C. Neil Hunter, and Klaus Schulten. Protein-induced membrane curvature investigated through molecular dynamics flexible fitting. Biophys. J., 97:321-329, 2009.
Johan Strümpfer and Klaus Schulten. Light harvesting complex II B850 excitation dynamics. Journal of Chemical Physics, 131:225101, 2009.
Johan Strümpfer, Melih Sener, and Klaus Schulten. How quantum coherence assists photosynthetic light harvesting. Journal of Physical Chemistry Letters, 3:536-542, 2012.



