Silica force field
Parameterizing amorphous silica surfaces
Since interactions with biomolecules in all present biotechnical applications of silica occur in an aqueous environment, an empirical force field for silica should reproduce its surface wetting properties. In computer simulations, the wetting (or hydrophobicity) is determined by the potential energy function of the system, the force field, that returns the interacting forces between atoms as a function of their atomic coordinates.
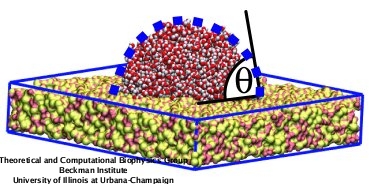
In a recent study, we developed a force field for amorphous silica surfaces based on their macroscopic wetting properties that is compatible with the CHARMM force field and TIP3P water model. The contact angle of a water droplet with silica served as a criterion to tune the intermolecular interactions. Figure 1 illustrates the water contact angle of a water droplet resting on a silica surface: the angle (&theta) is determined by the tangent of a water droplet and the solid surface.
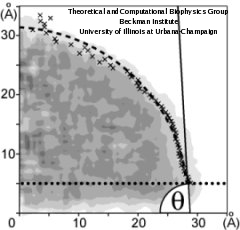
The calculation of the water contact angle is schematically presented in Figure 2. The density profile (gray tones) is computed using horizontal layers. To find the droplet boundary, each layer is fitted to the function.
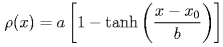
which represents the decrease of the radial density. Here &rho(x) is the radial density, a and b are proportional to the bulk liquid density and the thickness of the interface, respectively, and x0 is the layer boundary. The boundary x0 for each horizontal layer is pictured as an "x". The resulting boundary is fitted to a circular segment (dashed line), the boundary within 0.5 nm from the bottom being excluded (dotted line).
We have tested five models of silica surfaces (see Figure 3) with different surfaces modifications. For more details about the protocols to obtain silica structures, refer to our recent paper.
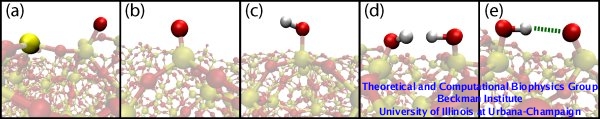 |
Figure 3. Five models for silica surfaces. All surfaces are composed of non-dangling atoms along with five different modifications: (a) Annealed surface with dangling silicon and oxygen atoms (opaque yellow and red atoms, respectively). (b) Surface of type I with only dangling oxygens (opaque red atom). (c) Surface of type II with silanols. (d) Surface of type III with viccinal silanols. (e) Surface type IV with deprotonated viccinal silanols, a hydrogen bond between each pair of silanols being enforced (green dashed line). |
Force Field Parameters
To parameterize a force field that can account for silica-water interactions and subsequently also for silica-biopolymer interactions, we employed a potential energy function compatible with the CHARMM force field. The functional form for the intermolecular interactions is given by:
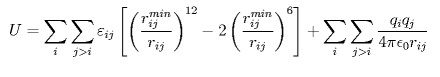
The first term on the right side describes the vdW interactions, which are represented through a Lennard-Jones 6-12 potential with two adjustable parameters: rimin, the distance at which the energy between atoms i and j is minimal, and &epsilonij, the well depth. The second term on the right side of the equation describes the electrostatic interactions, which are calculated using Coulomb's law. qi and qj are the partial atomic charges of atoms i and j, and &isin0 the vacuum permittivity.
The obtained force field parameters are summarized in Table 1. For annealed and type I surface (Figure 3.a and 3.b, respectively, see also movie 1) we found parameters that can reproduce the experimental silica wettability. Silica surfaces of type II, III and IV, which contain hydroxyl groups, produce complete wetting (&theta=0), independently of the magnitudes of the parameters and the hydroxyl concentration (see movie 2). For more details about the parameterization scheme and parameter values tested, refer to our recent paper.
Surface |
&epsilonSi(kcal/mol) |
&epsilonO(kcal/mol) |
rSimin(10-10m) |
rOmin(10-10m) |
qSi(|e|) |
qO(|e|) |
qDO(|e|) |
| annealed | 0.3 | 0.15 | 4.295 | 3.5 | 1.0 | -0.5 | |
| type I (first solution) | 0.3 | 0.15 | 4.295 | 3.5 | 0.9 | -0.45 | -0.044 |
| type I (second solution) | 0.3 | 0.15 | 4.295 | 3.5 | 0.9 | -0.45 | -0.423 |
Movies
 |
Movie 1. Equilibration of a water droplet on a hydrophobic annealed silica surface. (mpg 11M)The movie shows the equilibration of a drop composed of 1981 water molecules during 2 ns of MD simulation. The initial configuration corresponds to a water disc of 3 nm height and 6 nm diameter placed on top of an annealed silica slab of 11.4 nm x 114. nm x 2.0 nm dimension. |
 |
Movie 2. Silanol groups enhance surface wettability. (mpg 19M)The movie shows that the initial spreading of the water molecules over the surface of type II is being guided by the locations of surface silanols. Surface silanols are pictured in dark colors. Snapshots correspond to (a) 0, (b) 0.12, (c) 0.64, and (d) 2.0 ns of a MD simulation of a water disc (blue) resting on top of the silica surface with a silanol concentration of 1.0 nm-2. |
Publications
Investigators
Related TCB Group Projects
Page created and maintained by Eduardo R. Cruz-Chu.