Inter-Complex Excitation Transfer

Having arrived at the B850 BChl ring of LH-II the photon's excitation energy is transfer to the B875 BChl ring of LH-I and from there on to the reaction center. The excitation transfer LH-II -> LH-I -> RC occurs within 100 ps and with near unit efficiency. Time-resolved spectroscopy has revealed a 3-5 ps for the B850 -> LH-I transfer step. The final LH-I -> RC transfer step was measured to require 35 ps, making it the slowest step.
The structure of LH-II of Rs. molischianum has been resolved through a combination of x-ray crystallography and ab initio molecular modeling (see LH2 structure webpage ). Employing the same ab initio modeling protocol used for the elucidation of LH-II of Rs. molischianum , the structure of LH-I of Rb. sphaeroides has been modeled. LH-I has been docked to the photosynthetic reaction center of Rb. sphaeroides and surrounded by likewise modeled LH-II complexes of Rb. sphaeroides . Thus, for the first time, the complete structure of the photosynthetic unit of one species is available.
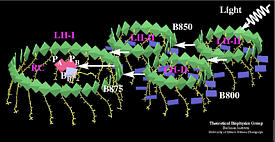 Excitation transfer in the bacterial photosynthetic unit.
LH-II contains two types of BChls commonly referred to as B800 (dark blue)
and B850 (green) which absorb at 800 nm and 850 nm respectively. BChls in
LH-I absorb at 875 nm, and are labeled as B875 (green).
PA and PB refer to the reaction center special pair, and BA, BB
to the accessory bacteriochlorophylls. The figure exhibits clearly the co-planar
arrangement of the B850 BChl ring in LH-II, the B875 BChl ring of LH-I, and the
reaction center BChls PA, PB, BA, BB. The figure has been produced with the program VMD .
Excitation transfer in the bacterial photosynthetic unit.
LH-II contains two types of BChls commonly referred to as B800 (dark blue)
and B850 (green) which absorb at 800 nm and 850 nm respectively. BChls in
LH-I absorb at 875 nm, and are labeled as B875 (green).
PA and PB refer to the reaction center special pair, and BA, BB
to the accessory bacteriochlorophylls. The figure exhibits clearly the co-planar
arrangement of the B850 BChl ring in LH-II, the B875 BChl ring of LH-I, and the
reaction center BChls PA, PB, BA, BB. The figure has been produced with the program VMD .
Click on the figure for a 91 kB version of the chromophore arrangement in the PSU
The most prominent features of the overall pigment organization is
the coplanar arrangement of the B850 BChls of LH-II,
the B875 BChls of LH-I, as well as the BChls inside the RC.
Such a planar organization of BChls in the bacterial
photosynthetic unit is optimal for energy transfers based on the delocalized exciton model.
The availability of an atomic scale model of the complete photosynthetic unit opens the way for structure-based calculations of energy transfer. An effective Hamiltonian can be constructed that describes the exciton system of the entire aggregate.
Using a perturbation scheme, the effective Hamiltonian allows one
to determine the rates of transfer of electronic excitation between LH-IIs, between LH-II and LH-I, and between LH-I and RC. The calculated time constants of 3.3 and 65 ps for the excitation transfer
processes LH-II -> LH-I and LH-I -> RC in Rb. sphaeroides , respectively, are in agreement with experimental values of 3-5 ps and 35 ps.
A startling result from these calculations has been a suggested role of the accessory BChls as mediators of the excitation transfer from LH-I to the RC special pair: the calculated time for LH-I ->RC transfer, in the absence of accessory BChls, is about 600 ps, which is an order of magnitude too long compared with observations; the accessory BChls in RC provide a tunneling path for the excitation transfer which bridges the large distance of 42 Angstroms or longer between LH-I BChls and the RC special pair.
Relevant Publications



