Lac Repressor-DNA Complex
 Structure of the Lac Repressor
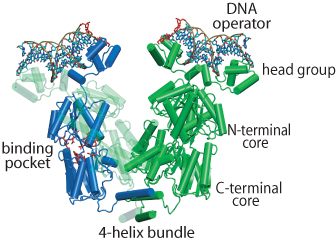
Structure of the Lac Repressor
The regulation of lac operon — a set of E. Coli genes responsible for the lactose metabolism in the bacteria — is a classic paradigm of genetic control. A key to controlling the operon is the DNA-binding protein called the lac repressor (LacI), shown on the left. In the absence of lactose, LacI inhibits the expression of the operon by binding to two out of three operator sites and causing the DNA between the bound sites to fold into a loop. There are three operators that LacI can bind: O1, O2 and O3. Depending on which operators are bound, the loop may have a length of either 384 or 75 base pairs. When lactose becomes available in the environment as the energy source, it can bind into a pocket inside LacI. The repressor lets go of the DNA and the genes become expressed again.
LacI is a tetrameric protein, that can be thought of as a "dimer of dimers", connected by a bundle of 4 alpha helices. Each dimer consists of two 360 residue-long monomers tightly folded together and is morphologically divided into (1) a head group, which is the DNA binding domain, (2) a core which contains the lactose binding site between its N-terminal and C-terminal halves, and (3) the end helix that forms the 4-helix bundle when the protein is tetramerized. The helical bundle can act like a hinge for the dimeric "hands". LacI attaches itself to the genomic DNA so that each dimer binds a separate operator site. The DNA between the operators becomes folded into a loop. Several X-ray and NMR structures of LacI have been solved, but none of them contained all-atom information for the whole protein. We built an all-atom structure of LacI based on available PDB structures and molecular modeling and deposited the resulting structure to PDB (code 1Z04).
It is notable that both the modeled structure of the LacI-DNA complex, as well as the experimental structures, include only disjoint protein-bound DNA segments, not connected by a DNA loop. However, the DNA loop would resist bending, and the force of its resistance is likely to change the LacI structure and dynamics. It is therefore very interesting to discern the mechanisms and the degree of such changes, elucidating the interactions that occur in the genome of a real bacterium (as opposite to the reduced systems used in the X-ray/NMR studies). The structure and dynamics of the folded loop is also important for understanding the gene regulation process, because the folded loop contains binding sites for other proteins actively involved in gene expression (RNA polymerase, catabolite gene activator). However, an all-atom model system including the DNA loop would be exceedingly large and the simulations of it on modern-day computers would be too slow. Besides, in this system one deals with molecular events occurring on very different time scales (the fast structural dynamics of the protein vs the slow relaxational dynamics of the DNA loop). This exciting system, therefore, presents an ideal case for our multiresolution approach.
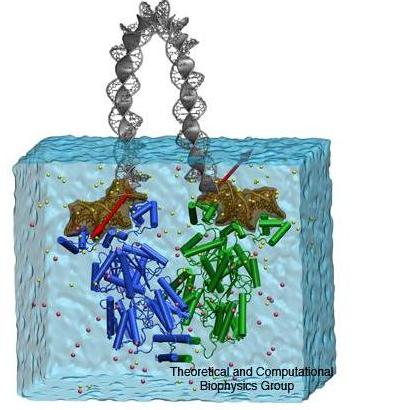 Multiscale simulation of LacI-DNA complex.
Multiscale simulation of LacI-DNA complex.
Structural Dynamics of the LacI-DNA Complex
The multiscale approach, used to study the structural dynamics of the LacI complex with the 75 bp DNA loop has been described in our recent publication. In short, the all-atom model of the protein with the DNA segments bound to the head groups was solvated in a bath of water and ions. The molecular dynamics (MD) of that system was studied with NAMD. The DNA loop connecting the protein-bound segment was described using a mathematical model which approximated the DNA by an electrically charged elastic rod. The boundaries of the rod were defined by the instantaneous positions of the ends of the protein-bound operator segments. The force of the loop resistance to bending was included in the MD simulations via special force terms acting on the protein-bound segments. Thus the changes in the protein structure occurring under the strain of the bent DNA on the simulation time scale could be observed.
The Head Groups Absorb All The Strain Arising From The DNA Loop
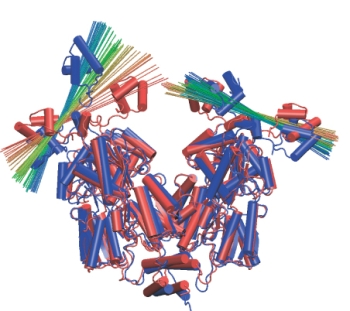 Head group rotation. A line is drawn across each head group to show its
orientation over time. Red corresponds to the beginning of the simulation,
blue to the end.
Head group rotation. A line is drawn across each head group to show its
orientation over time. Red corresponds to the beginning of the simulation,
blue to the end.
 The change in the loop structure between the beginning (yellow) and the end
(green) of the simulation accounts for the experimentally observed change
in the distance between the fluorophores attached near the ends of the protein-bound DNA segments.
The change in the loop structure between the beginning (yellow) and the end
(green) of the simulation accounts for the experimentally observed change
in the distance between the fluorophores attached near the ends of the protein-bound DNA segments.
During the simulation, the protein domains behaved very much like rigid bodies moving with respect to each other. These motions were small and random during the control simulation that did not include the forces from the DNA loop. In the simulations that did include the forces from the loop, the domain motions increased in scale, especially those of the head groups. The head groups rotated in different directions with respect to their dimer core domains, as shown on the left. In contrast, the cleft between the LacI dimers showed only an insignificant increase. The loop structure was much relaxed during the simulation, its energy dropped from ~20kT to ~12kT. Thus, solely the rotation of the head groups is able to absorb much of the strain arising from the DNA loop, allowing LacI to wrestle DNA without losing its grip on it. This is a very interesting finding, because up to now it was believed that it would be easy to open the protein by rotating its dimer "hands" around the 4-helix bundle, making such rotation the principal degree of freedom of the protein. A beautiful experiment measured the distance between fluorophores attached to the DNA loop near the lac repressor head groups to be ~5nm larger than the same distance in the crystal structure. We could measure a very similar increase in distance in our simulation (see Figure on the right), thus explaining the experimental observations without invoking any significant opening of the cleft!
Watch a movie of the simulation here (Quicktime required, [an error occurred while processing this directive])
Opening LacI: The Protein Lock
The results of the multiscale simulation described above were unexpected: the protein "hands" did not open easily, contrary to what had been thought. In another simulation, we forced the opening of the protein by applying extra forces to the ends of the protein-bound DNA. This forced pulling mimicked single molecule experiments such as atomic force microscopy or optical tweezers. The goal was to understand what interactions kept the lacI dimers in their initial "V" configuration, preventing an opening of the cleft between them. The figure below shows snapshots of the protein during the cleft-opening simulation.
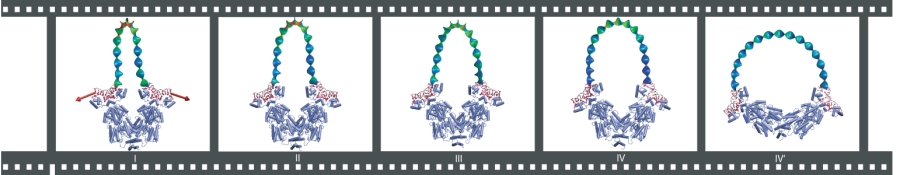 Opening
simulation of LacI-DNA complex. Forces are applied to the ends of the
protein-bound DNA segments in order to mimic the opening of the lac
repressor in vivo and in
experiment. The color scale of the elastic rod model of DNA
corresponds to the energy stored along the loop (red showing high
density of elastic energy, and blue, low energy density).
Opening
simulation of LacI-DNA complex. Forces are applied to the ends of the
protein-bound DNA segments in order to mimic the opening of the lac
repressor in vivo and in
experiment. The color scale of the elastic rod model of DNA
corresponds to the energy stored along the loop (red showing high
density of elastic energy, and blue, low energy density).
Watch a movie of the opening here (Quicktime required, 2.4M)
The simulations revealed fascinating details of the mechanism of
repression: when LacI is subject to strain from the DNA loop, a
"lock" mechanism kicks in and prevents the cleft from opening. The
locking interactions mainly occur between three groups of protein
residues, which account for 80% of the dimer-to-dimer binding
energy (excluding the energy of the 4-helix bundle).
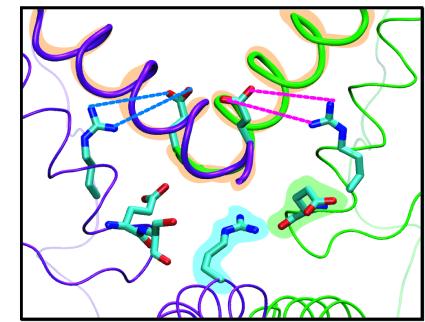 The lock of LacI. Three groups of interacting residues keep LacI in the "V"
configuration when wrestling the DNA loop.
The lock of LacI. Three groups of interacting residues keep LacI in the "V"
configuration when wrestling the DNA loop.
The first interaction (orange in figure) is the attraction between the adjacent ends of the two alpha helices connecting the dimers. This interaction is the only one seen in the crystal structure or in the control simulations of the LacI without the DNA loop.
The second interaction is caused by the salt bridges R326:2A-E259:1B (blue) and R326:1A-E259:2B (magenta). These salt bridges do not form unless LacI is subject to strain thereby yielding a small pre-opening of the cleft. The salt bridges are very hard to break, and contribute to the stability of the "V" configuration.
Finally, R351:1A interacts with the dipole moment of a helix from either the other dimer (green) or its own dimer (purple). During the opening of the cleft, the dimers gradually move away from each other, causing R351 to switch to the helix in its own dimer.
These interactions unravel a mystery surrounding LacI dynamics. The mutations of the key residues shall weaken the protein lock and lower the force barrier required to open the cleft in the micromanipulation experiments.
Watch a movie of opening the protein "lock" here (Quicktime required, 3.0M)
To Learn More...
Read the article by Trish Barker in NCSA's Access Magazine.
Publications
Investigators
Page created and maintained by Elizabeth Villa.



