Research Topics - Bioenergetics
Photosynthetic complexes provide fascinating challenges to biophysicists. With the availability of recent atomic structures for protein-pigment complexes such as photosystem I in cyanobacteria and plants, it is possible to form a comprehensive picture of the light absorption and excitation migration processes based on an atomic level quantum mechanical description. This kind of structural analysis not only provides a rigorous test for our understanding of the physics of these mechanisms through a comparison to spectroscopy and kinetics experiments, but it also forms a framework within which the organizational principles for multi-component pigment-protein assemblies can be investigated.
Photosynthetic units (PSUs) of living organisms are complex assemblies of several multi-protein aggregates. We constructed the structure of the PSU of purple bacteria. The components involved were the crystal structure of the bacterial photosynthetic reaction center surrounded by the model structure of the light-harvesting complex I and several structures of the light-harvesting complex II, solved in a collaborative project with X-ray crystallographers. The constructed structure is used to study the light energy transfer driving the bacterial photosynthesis.
Papers
Zooming in on ATP hydrolysis in F1. Markus Dittrich and Klaus Schulten. Journal of Bioenergetics and Biomembranes, 37:441-444, 2005.
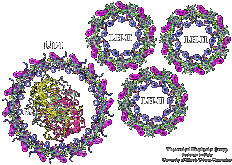
Comparison of the light harvesting networks of plant and cyanobacterial photosystem I. Melih K. Sener, Craig Jolley, Adam Ben-Shem, Petra Fromme, Nathan Nelson, Roberta Croce, and Klaus Schulten. Biophysical Journal, 89:1630-1642, 2005.
When light falls in LOV: A quantum mechanical/molecular mechanical study of photoexcitation in Phot-LOV1 of Chlamydomonas Reinhardtii. Markus Dittrich, Peter L. Freddolino, and Klaus Schulten. Journal of Physical Chemistry B, 109:13006-13013, 2005.
Physical principles of efficient excitation transfer in light harvesting. Melih Sener and Klaus Schulten. In David L. Andrews, editor, Energy Harvesting Materials, pp. 1-26. World Scientific, Singapore, 2005.
Role of water in transient cytochrome c2 docking. Felix Autenrieth, Emad Tajkhorshid, Klaus Schulten, and Zaida Luthey-Schulten. Journal of Physical Chemistry B, 108:20376-20387, 2004.
ATP hydrolysis in the βTP and βDP catalytic sites of F1-ATPase. Markus Dittrich, Shigehiko Hayashi, and Klaus Schulten. Biophysical Journal, 87:2954-2967, 2004.
Role of hydrogen-bond network in energy storage of bacteriorhodopsin's light-driven proton pump revealed by ab initio normal mode analysis. Shigehiko Hayashi, Emad Tajkhorshid, Hideki Kandori, and Klaus Schulten. Journal of the American Chemical Society, 126:10516-10517, 2004.
Excitation migration in trimeric cyanobacterial photosystem I. Melih K. Sener, Sanghyun Park, Deyu Lu, Ana Damjanović, Thorsten Ritz, Petra Fromme, and Klaus Schulten. Journal of Chemical Physics, 120:11183-11195, 2004.
Molecular dynamics simulation of bacteriorhodopsin's photoisomerization using ab initio forces for the excited chromophore. Shigehiko Hayashi, Emad Tajkhorshid, and Klaus Schulten. Biophysical Journal, 85:1440-1449, 2003.
Molecular dynamics investigation of primary photoinduced events in the activation of rhodopsin. Jan Saam, Emad Tajkhorshid, Shigehiko Hayashi, and Klaus Schulten. Biophysical Journal, 83:3097-3112, 2002.