The Tale of Two Photosystems
Photosystem I (PSI) is a membrane protein that is one of two major reaction center complexes utilized by oxygenic photosynthetic organisms, such as cyanobacteria, green algae, or higher plants. PSI uses electronic excitation energy resulting from the absorption of sunlight for transporting electrons across the membrane. The charge gradient thus created is later transformed into the energy in chemical bonds in a series of steps by which incident light energy is stored in progressively more stable forms. The structure and function of photosystem I, especially in the context of the cyanobacterial system, is discussed further at our organization of energy transfer networks page.
Below we present in further detail a comparison of the light-harvesting networks of plant and cyanobacterial photosystem I described in a recent paper. The aim of this study is a glimpse at the physical principles that shaped the evolution of this ubiquitous photosynthetic complex.
Distant cousins: plants and cyanobacteria
Photosynthesis performed by modern plants has its evolutionary origins in cyanobacterial systems. Chloroplasts in plants, where photosynthesis is performed and where photosystem I resides, have evolved through the endosymbiosis of cyanobacteria with eukaryotic cells over a billion years ago. Thus, a comparison between photosystem I structures from plants and from cyanobacteria as presented below captures the consequences of divergent evolution over a long time.
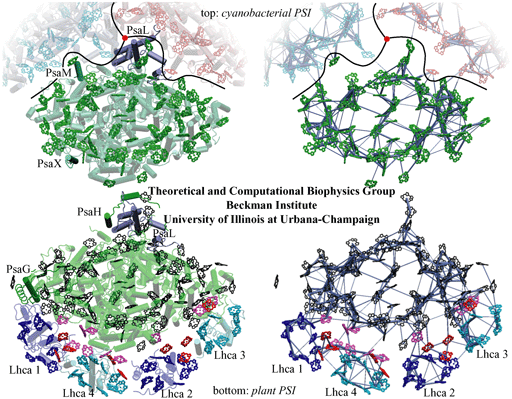
Comparison of cyanobacterial and plant photosystem I structures and corresponding excitation transfer networks. (top) Top view of cyanobacterial photosystem I. The relative positions of two additional photosystem I monomers (in blue and red) in a trimer as well as the trimer axis (red disk) are indicated. Subunits PsaM and PsaX, which are unique to cyanobacteria, and the subunit PsaL, located near the trimer axis, are highlighted. (bottom) Top view of the plant PSI-LHCI supercomplex. Subunits PsaG and PsaH, which are unique to plants, are highlighted, along with subunit PsaL. Lhca subunits and their associated chlorophylls are represented in blue (Lhca 1 and 2) and cyan (Lhca 3 and 4).
The figures on the right depict the excitation transfer pathways in the chlorophyll networks of cyanobacterial (top) and plant (bottom) photosystem I. The thickness of a bond between two pigments is proportional to log(t)+c, where c is a constant and t is the transfer rate between pigments. The highest of the forward and backward transfer rates is chosen.
A notable difference of the plant photosystem I complex from its cyanobacterial counterpart is the presence of four accompanying Light Harvesting Complex (LHC) subunits, named Lhca1-4 after their encoding genes. These subunits and the "gap" region that connects them to the photosystem I core feature extra chlorophylls bringing the total chlorophyll count of plant photosystem I to 167, up from 96 in cyanobacteria.
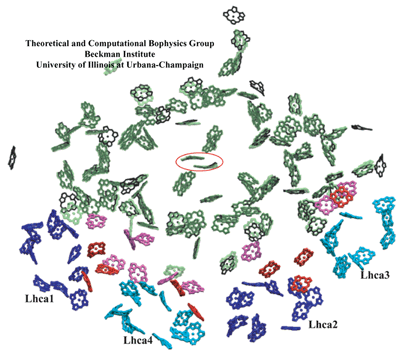 Figure made with VMD.
Figure made with VMD.
Chlorophyll clusters of PSI. (green, transparent) Cyanobacterial PSI chlorophylls; (black) plant PSI core chlorophylls; and (pink) plant PSI "gap chlorophylls" situated between core chlorophylls and Lhca chlorophylls. (blue and cyan) Chlorophylls associated with plant Lhca subunits 1, 4, 2, and 3, alternatingly; and (red) plant Lhca "linker chlorophylls". Eighty-one of the original 96 chlorophylls in cyanobacterial photosystem I have a corresponding chromophore in the plant system located within 1 Angstrom. The special pair chlorophylls, where charge separation is initiated, are indicated by a surrounding red ellipse. For the sake of simplicity, chlorophylls are represented only by their chlorine rings. Some Lhca chlorophylls are shown as porphyrins since their orientations were not determined unambiguously in the structural model.
The high degree of conservation observed especially at the central region of photosystem I between plants and cyanobacteria suggests the role of strong constraints that act upon the photosynthesis process preventing further changes to the pigment geometry.
Excitation transfer kinetics in plant photosystem I
A first test of any structure based kinetic model should be reproducing the observed characteristics of the excitation transfer kinetics. Similar to earlier studies for the cyanobacterial system, a detailed picture of room temperature excitation transfer processes is obtained in the context of an effective Hamiltonian formulation. In particular, an average excitation lifetime of about 49 ps was reported on the basis of the theoretical work well in agreement with observations.
The excitation transfer kinetics in plant photosystem I has some features that set it apart from cyanobacteria due to the presence of plant-only chlorophyll clusters.
Connectivity of the outer subunits in plant photosystem I
It is to be expected that the additional chlorophyll clusters found in plant photosystem I are efficiently coupled to the core in order to maintain a high quantum yield of the system (see the page "Organization of energy transfer networks" for a definition of quantum yield). Indeed, the excitation transfer networks depicted above suggest already an efficient integration of the Lhca chlorophyll groups. However, it is possible in principle to maintain a high quantum yield while still allowing for a separation of timescales for excitation transfer between the Lhca and core chlorophyll clusters. The figure below illustrates that the timescales for excitation delivery times from Lhca subunits (solid circles) are actually not separated in magnitude from the delivery times for core chlorophylls (open circles).
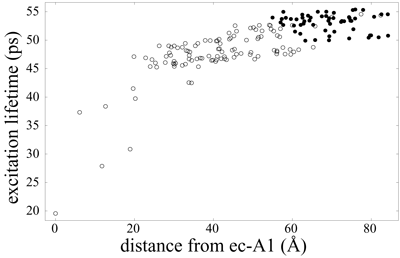
Average excitation lifetime as a function of the distance of the starting chlorophyll from the reaction center chlorophyll ec-A1. Open circles represent PSI core and gap chlorophylls, whereas solid circles represent Lhca and linker chlorophylls. Excitation lifetimes for outer chlorophylls are seen to be not much higher than those for core chlorophylls, indicating an efficient coupling of Lhca subunits to the core. The mean lifetime averaged over all Lhca subunits (including linker chlorophylls) is 53.1 ps and 47.6 ps when averaged over the PSI core (including gap chlorophylls). The overall average excitation lifetime of the PSI-LHCI supercomplex is 49.4 ps.
The kinetic model thus introduced also permits the computation of transfer times between various chlorophyll clusters that constitute plant photosystem I as illustrated below. The presence of gap chlorophylls situated between Lhca and core chlorophyll clusters is essential for the rapid transfer times observed.
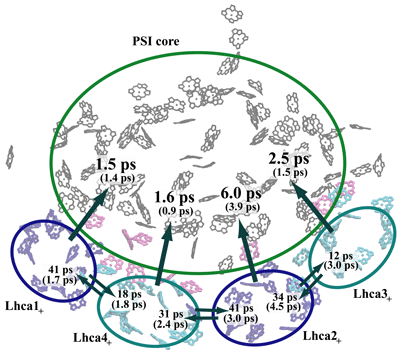
Transfer times (reciprocal of transfer rates) between the Lhca subunits and the PSI core. The subscript "+" of the Lhca subunits indicates that the neighboring linker chlorophylls belonging to the same chain are included with a given subunit. A Boltzmann equilibrium of donor states is assumed before transfer. The first number corresponds to a spectrally refined hybrid Hamiltonian, whereas the second number (in parentheses) corresponds to a uniform site energy distribution for non-conserved chlorophylls. We conclude that inter-Lhca transfer rates cannot be assigned reliably due to the sensitivity of these values on site energy (excitation energy of individual chlorophylls) assignments. The collective transfer time from all Lhca subunits to the PSI core (including gap chlorophylls) is 2.1 ps (becoming 1.5 ps in the uniform site energy model). The transfer time from the chlorophyll-b forms to the chlorophyll-a pool of Lhca subunits, computed similarly, is 288 fs.
Evolution of the chlorophyll network
The evolution of a biological network is shaped by a hierarchy of physical constraints that act upon its dynamics. However, there are two major challenges in relating network organization and function to network evolution. First, accidental events play a major role during evolution. Thus, any model of network evolution has to be stochastic rather than deterministic. Second, empirical data regarding the state of a biological network during different stages of evolution are hard to obtain. In this regard, light-harvesting complexes and their associated excitation transfer networks provide a unique opportunity, where the geometry of a biological network and a detailed quantum mechanical description of its dynamics can be compared for closely related species as presented above.
It was suggested earlier that perhaps maintaining a high efficiency for the excitation transfer process may be a constraint that plays a role in shaping the network evolution. It was also argued that this is not a high degree constraint as it does not represent a rate limiting step. It is still to be determined conclusively whether minute changes (on the order of a percent or less) in the efficiency of one cellular process, such as light absorption and subsequent excitation transfer, play a determining role in shaping the evolution of an organism.
A comparison between plant and cyanobacterial photosystem I chlorophyll clusters suggests (we refer the reader to the publication for a more detailed discussion) that the orientations of the added outer chlorophylls do not further optimize an already high quantum yield and are practically indistinguishable from a set of directions randomly chosen over the unit sphere. However, the tight packing density of chlorophylls is still notable and is largely responsible for the high quantum yield. This, however, renders the near exact conservation of 81 central chlorophylls puzzling as only a small number of them have favorable orientations that further optimize the quantum yield. We suggest, therefore, that constraints other than excitation transfer, such as ligation of chlorophylls, electron transfer, photoprotection of chlorophylls by carotenoids, spectral composition of pigments, and the requirements of assembly, and possibly repair, are likely to play determining roles in shaping the evolution of a light-harvesting system. The apparent lack of optimality of the geometry of the peripheral chlorophyll network suggests then that the aforementioned issues display a higher priority for the fitness of the system than the excitation transfer process.
Quantitative models of network evolution are very elusive presently. The availability of more structural information from related species as well as the conceptual development of a quantifiable hierarchy of constraints will be necessary components in understanding the evolutionary dynamics of biological networks, photosynthetic energy transfer networks being one example.
Related links
- Organization of energy transfer networks
A more introductory page discussing the fundamentals of the light absorption and energy transfer processes in the context of the robustness and optimality of the chlorophyll network. - Quantum Biology of the Photosynthetic Unit
- Light-Harvesting by Carotenoids
Related Publications
People
- Melih Sener
- Craig Jolley
- Adam Ben-Shem
- Petra Fromme
- Nathan Nelson
- Roberta Croce
- Klaus Schulten