|
Which residues are most involved in the process of binding/unbinding? How
large are the conformational changes induced in the protein by the
binding/unbinding process? Can the hormone use one pathway for binding and
another pathway for unbinding? To answer these questions, the unbinding of
retinoic acid from its receptor was induced by applying an external force on the
hormone.
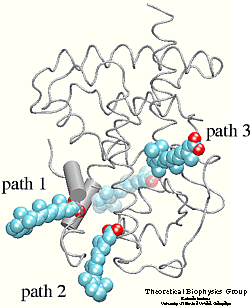 The crystal structure of the human retinoic acid
receptor (hRAR)-gamma bound to all-trans retinoic acid (t-RA) suggests that
the possible entry point for the hormone is closed by helix 12. Renaud et al.
proposed that when t-RA binds to the receptor, helix H12 changes its
conformation from extended into solvent, as seen in the crystal structure of
retinoid X receptor (RXR) , to a conformation
closing the entrance to the binding pocket as in the crystal structure of
retinoic acid receptor (RAR) . Analysis of the
surface of the protein identified another possible entry point: the only ``window'' in the surface of the protein that allows
the hormone to be seen. Three possible unbinding pathways were studied. Path 1
and path 2 were chosen in the proximity of helix H11 and H12. Path 3 was chosen
to study the unbinding of the hormone through the
``window'' .
The crystal structure of the human retinoic acid
receptor (hRAR)-gamma bound to all-trans retinoic acid (t-RA) suggests that
the possible entry point for the hormone is closed by helix 12. Renaud et al.
proposed that when t-RA binds to the receptor, helix H12 changes its
conformation from extended into solvent, as seen in the crystal structure of
retinoid X receptor (RXR) , to a conformation
closing the entrance to the binding pocket as in the crystal structure of
retinoic acid receptor (RAR) . Analysis of the
surface of the protein identified another possible entry point: the only ``window'' in the surface of the protein that allows
the hormone to be seen. Three possible unbinding pathways were studied. Path 1
and path 2 were chosen in the proximity of helix H11 and H12. Path 3 was chosen
to study the unbinding of the hormone through the
``window'' .
Simulation results indicated that it is possible to
unbind the hormone along path 1 and path 3 without greatly affecting
the structure of the protein. Particular characteristics of the
simulated pathways suggest path 1 as the binding
pathway for the hormone and path 3 as the unbinding pathway . Using the reversed order of events observed in the simulated unbinding along
path 1 one can describe the binding mechanism of the hormone. First, Arg413, and
then Arg396 attract and orient the carboxylate end of the hormone towards
the binding pocket. When the hormone is within hydrogen bonding
distance of these two residues, it experiences also the influence of
the charged and polar residues Arg278, Lys236, Arg274, and Ser289
located at the opposite end of the binding pocket.
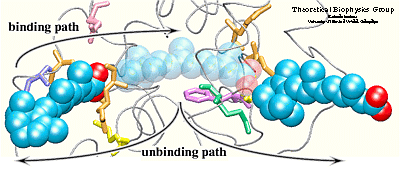 The two steps
described above take place almost simultaneously, i.e., the strong
electrostatic attraction between the carboxylate end of the hormone
and residues Arg278, Lys236, Ser289, and Arg 274 helps the
beta-ionone ring to pass between protein residues, thus, leading
to the penetration of the hormone into the binding pocket. The
entrance of path 1 is surrounded by highly fluctuating residues. These
residues may become more ordered upon binding of the hormone, making
contacts with the hormone or with other protein residues. Induced
ordering of the protein side chains may be favorable for the hormone
entry without requiring a motion of helix H12.
Path 3 is an unlikely candidate for a binding pathway for the following reasons:
1) the carboxylate end of the hormone should be
the one to be attracted by the point of entry into the protein since
it furnishes stronger and more specific interactions than the
beta-ionone ring; 2) the ``window'' would need to be sufficiently
large to let the bulky ring pass through first. Therefore, one may then consider
path 3 as a possible unbinding pathway, either as the sole unbinding
pathway or an alternative to path 1.
The two steps
described above take place almost simultaneously, i.e., the strong
electrostatic attraction between the carboxylate end of the hormone
and residues Arg278, Lys236, Ser289, and Arg 274 helps the
beta-ionone ring to pass between protein residues, thus, leading
to the penetration of the hormone into the binding pocket. The
entrance of path 1 is surrounded by highly fluctuating residues. These
residues may become more ordered upon binding of the hormone, making
contacts with the hormone or with other protein residues. Induced
ordering of the protein side chains may be favorable for the hormone
entry without requiring a motion of helix H12.
Path 3 is an unlikely candidate for a binding pathway for the following reasons:
1) the carboxylate end of the hormone should be
the one to be attracted by the point of entry into the protein since
it furnishes stronger and more specific interactions than the
beta-ionone ring; 2) the ``window'' would need to be sufficiently
large to let the bulky ring pass through first. Therefore, one may then consider
path 3 as a possible unbinding pathway, either as the sole unbinding
pathway or an alternative to path 1.
Movies for the SMD simulations
Publications
- [1] D. Kosztin,
S. Izrailev and K. Schulten. Unbinding of retinoic acid from its receptor
studied by Steered Molecular Dynamics. Biophysical J., 76:188--197, 1999
|

 The crystal structure of the
The crystal structure of the  The two steps
described above take place almost simultaneously, i.e., the strong
electrostatic attraction between the carboxylate end of the hormone
and residues Arg278, Lys236, Ser289, and Arg 274 helps the
beta-ionone ring to pass between protein residues, thus, leading
to the penetration of the hormone into the binding pocket. The
entrance of path 1 is surrounded by highly fluctuating residues. These
residues may become more ordered upon binding of the hormone, making
contacts with the hormone or with other protein residues. Induced
ordering of the protein side chains may be favorable for the hormone
entry without requiring a motion of helix H12.
Path 3 is an unlikely candidate for a binding pathway for the following reasons:
1) the carboxylate end of the hormone should be
the one to be attracted by the point of entry into the protein since
it furnishes stronger and more specific interactions than the
beta-ionone ring; 2) the ``window'' would need to be sufficiently
large to let the bulky ring pass through first. Therefore, one may then consider
path 3 as a possible unbinding pathway, either as the sole unbinding
pathway or an alternative to path 1.
The two steps
described above take place almost simultaneously, i.e., the strong
electrostatic attraction between the carboxylate end of the hormone
and residues Arg278, Lys236, Ser289, and Arg 274 helps the
beta-ionone ring to pass between protein residues, thus, leading
to the penetration of the hormone into the binding pocket. The
entrance of path 1 is surrounded by highly fluctuating residues. These
residues may become more ordered upon binding of the hormone, making
contacts with the hormone or with other protein residues. Induced
ordering of the protein side chains may be favorable for the hormone
entry without requiring a motion of helix H12.
Path 3 is an unlikely candidate for a binding pathway for the following reasons:
1) the carboxylate end of the hormone should be
the one to be attracted by the point of entry into the protein since
it furnishes stronger and more specific interactions than the
beta-ionone ring; 2) the ``window'' would need to be sufficiently
large to let the bulky ring pass through first. Therefore, one may then consider
path 3 as a possible unbinding pathway, either as the sole unbinding
pathway or an alternative to path 1.


