C&S4: Ribosomal Holo-translocon Supercomplex
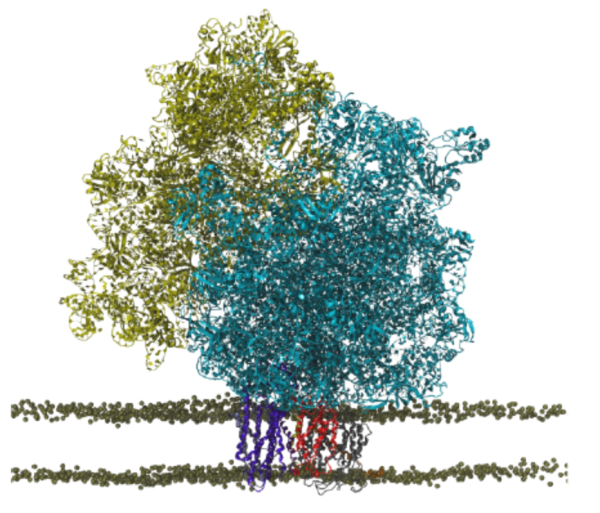
For most membrane proteins, the process of insertion and integration into lipid membranes takes place concurrent with their translation and synthesis by the ribosome. Given their therapeutic relevance, the insertion pathways of nascent trans-membrane proteins into lipid membranes deserve more attention than they have hitherto received. The ribosomal insertion machinery consists mainly of YidC, SecY and the holo-translocon supercomplex; the holo-translocon supercomplex itself is composed of YidC, SecY and SecDF. Depending on size and nature of the inserting nascent protein, the membrane insertion process is performed by either YidC or SecY alone, or by both of them working in concert within the holo-translocon supercomplex. Here, we plan to (1) model an all-atom structure of a membrane-embedded ribosome-bound holo-translocon supercomplex, and then (2) investigate how conformational changes of the holo-translocon supercomplex regulates membrane proteins insertion pathways.
Most experimental probes fail to catch non-stationary states of non-equilibrium processes such as nascent protein insertion from the ribosome into the membrane. Computational techniques have proved instrumental in investigating such processes. A detailed investigation of protein insertion pathways via the holo-translocon, which necessitates the application of MD simulations, requires several difficult steps. The first involves building an all-atom structure, based on a low-resolution (8-10 Å) cryo-EM density map, for a membrane-embedded ribosome-bound holo-translocon. Center-developed hybrid modeling tools (TRD3) combining de novo structure prediction with MDFF will be employed to address the structure analysis step for a system of ~4 M atoms. Once the all-atom structure is available and simulated, the second difficulty is the millisecond timescale characteristic for protein insertion by the holo-translocon. The enhanced sampling features of NAMD (TRD1) permit one to overcome the timescale problem.
In collaboration with Schaffitzel, a detailed all-atom model of an isolated holo-translocon (without the ribosome) has already been determined employing MDFF (TRD3), a hybrid modeling tool developed at the Center. The stability of the model in a realistic membrane environment was assessed over the course of a μs MD trajectory. The MD simulation of the holo-translocon in the membrane further revealed the key residues that stabilize the holo-translocon supercomplex and revealed also how the inherent flexibility of the supercomplex allows it to adapt its conformation to the requirements of its protein substrates. The computationally identified key residues are currently being tested with mutational experiments (Schaffitzel) to confirm their individual and cumulative effects on the stability of the holo-translocon.
Having the isolated holo-translocon model available, in the upcoming research a ribosome-bound model of the holo-translocon in a membrane will be constructed. Representing the holo-translocon in its natural environment will provide a viable starting point for studying the holo-translocon mediated protein insertion pathway. In particular, the insertion pathway of a FoC helix will be probed; the FoC helix makes up the rotary motor of ATP synthase. In analogy to prior work on SecY, steered MD simulations will be employed to derive a preliminary FoC insertion pathway. Further refinements of this pathway will be accomplished utilizing enhanced sampling features of NAMD (TRD1). Similar to an earlier successful experimental-computational collaboration with the Beckmann group on YidC, the experimental collaborator (Schaffitzel) will perform cross-linking studies to confirm the computationally predicted relative position of FoC with respect to the constituent proteins of the holo-translocon, and particularly with respect to YidC which is expected to provide an exit way to FoC into the membrane. Our prior work on isolated YidC and SecY as well as colaborating with a world-leading experimental group offers the unique opportunity to probe how YidC and SecY work together within the holo-translocon supercomplex, and, thus, compare the key features of the three most ubiquitous pathways of membrane protein insertion.
