

Next: Missing Parameter Development
Up: VMD Tutorial
Previous: HisH System Setup
The form of the potential energy function we will use in
this exercise is taken directly from CHARMM22 and given by the
following equation [1]:
The first term in the energy function accounts for the bond
stretches where 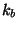 is the bond force constant and
is the bond force constant and 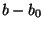 is
the distance from equilibrium that the atom has moved. The second
term in the equation accounts for the bond angles where
is
the distance from equilibrium that the atom has moved. The second
term in the equation accounts for the bond angles where 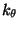 is the angle force constant and
is the angle force constant and
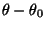 is the angle
from equilibrium between 3 bonded atoms. The third term is for the
dihedrals (a.k.a. torsion angles) where
is the angle
from equilibrium between 3 bonded atoms. The third term is for the
dihedrals (a.k.a. torsion angles) where 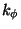 is the dihedral
force constant,
is the dihedral
force constant, 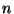 is the multiplicity of the function,
is the multiplicity of the function, 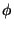 is
the dihedral angle and
is
the dihedral angle and 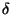 is the phase shift. The fourth
term accounts for the impropers (i.e. out of plane bending), where
is the phase shift. The fourth
term accounts for the impropers (i.e. out of plane bending), where
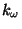 is the force constant and
is the force constant and
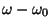 is the out
of plane angle. The Urey-Bradley component (cross-term accounting
for angle bending using 1,3 nonbonded interactions) comprises the
fifth term, where
is the out
of plane angle. The Urey-Bradley component (cross-term accounting
for angle bending using 1,3 nonbonded interactions) comprises the
fifth term, where 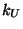 is the respective force constant and
is the respective force constant and 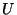 is the distance between the 1,3 atoms in the harmonic potential.
Nonbonded interactions between pairs of atoms
is the distance between the 1,3 atoms in the harmonic potential.
Nonbonded interactions between pairs of atoms 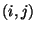 are
represented by the last two terms. By definition, the nonbonded
forces are only applied to atom pairs separated by at least three
bonds. The VDW energy is calculated with a standard 12-6
Lennard-Jones potential and the electrostatic energy with a
Coulombic potential.
are
represented by the last two terms. By definition, the nonbonded
forces are only applied to atom pairs separated by at least three
bonds. The VDW energy is calculated with a standard 12-6
Lennard-Jones potential and the electrostatic energy with a
Coulombic potential.


Next: Missing Parameter Development
Up: VMD Tutorial
Previous: HisH System Setup
vmd@ks.uiuc.edu
![$\displaystyle \sum_{\mathrm{bonds}}{k_b \left(b-b_0\right)^2} + \sum_{\mathrm{a...
...sum_{\mathrm{dihedrals}}{k_\phi \left[1+\cos\left(n\phi - \delta\right)\right]}$](img13.gif)
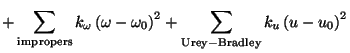
![$\displaystyle + \sum_{\mathrm{nonbonded}}{\epsilon \left[\left(\frac{R_{\mathrm...
...{R_{\mathrm{min}_{ij}}}{r_{ij}}\right)^6\right]+\frac{q_iq_j}{\epsilon r_{ij}}}$](img15.gif)
![$\displaystyle \sum_{\mathrm{bonds}}{k_b \left(b-b_0\right)^2} + \sum_{\mathrm{a...
...sum_{\mathrm{dihedrals}}{k_\phi \left[1+\cos\left(n\phi - \delta\right)\right]}$](img13.gif)
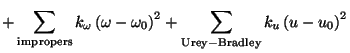
![$\displaystyle + \sum_{\mathrm{nonbonded}}{\epsilon \left[\left(\frac{R_{\mathrm...
...{R_{\mathrm{min}_{ij}}}{r_{ij}}\right)^6\right]+\frac{q_iq_j}{\epsilon r_{ij}}}$](img15.gif)
![\framebox[\textwidth]{
\begin{minipage}{.2\textwidth}
\includegraphics[width...
...ate the potential? What are the
typical barrier heights? }
\end{minipage}
}](img29.gif)