From: Radak, Brian K (bradak_at_anl.gov)
Date: Wed Oct 05 2016 - 15:39:01 CDT
In my opinion, if you want to quantify the fluctuations, you have to be careful in what you choose as your reference for RMSD - looking for anomalous domains, as you have, is probably an excellent idea (and much more informative than simply saying "the RMSD is noisy").
Long equilibration times are absolutely acceptable, especially if you are not so sure the initial conditions are physically realistic or relevant. Coming from the nucleic acids community we regularly expect equilibration times > 50 ns - I try to take people at their word when they say a protein system equilibrated faster than that, but it always seems overly fortuitous to me.
There are a number of analyses that might be useful for comparing with B factors - principle component analyses is probably the most popular. A cluster analyses is also probably a good idea (and might provide a better reference for RMSD calculations).
HTH,
Brian
Brian Radak
Postdoctoral Appointee
Leadership Computing Facility
Argonne National Laboratory
9700 South Cass Avenue, Bldg. 240
Argonne, IL 60439-4854
(630) 252-8643
brian.radak_at_anl.gov
________________________________
From: owner-namd-l_at_ks.uiuc.edu [owner-namd-l_at_ks.uiuc.edu] on behalf of Dhiraj Srivastava [dhirajks_at_gmail.com]
Sent: Wednesday, October 05, 2016 2:02 PM
To: namd-l_at_ks.uiuc.edu; Pardis Tabaee
Subject: Re: namd-l: question regarding rmsd
Thank you Everyone.
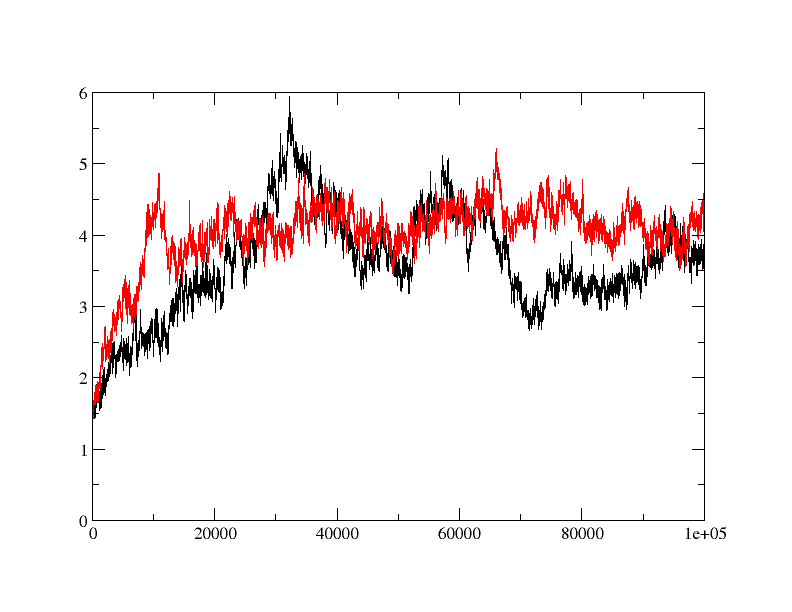
The simulation was started from crystal structure obtained by cocrystallizing with ligand. I don't see any significant difference in apo vs ligand bound form but ligand is an inhibitor. I think for unstable RMSD, I can blame a small flexible domain. After removing the flexible domain from RMSD calculation, I got stable RMSD very similar to apo protein. However its still took long time to equilibrate. the sudden change in rmsd at around 30 ns is still there. can I use data after 40 ns for the analysis? is it acceptable to have such a long equilibration time? I am primarily a crystallographer and I don't have much experience in publishing articles with MD simulation data. So I don't know what's acceptable in the community? I am doing simulation to find out if allosteric behaviour of ligand is due to change in dynamics. I would like to compare it with B factor change in crystal structure.
Thanks
Dhiraj
On Wed, Oct 5, 2016 at 1:34 PM, Pardis Tabaee <pardis.tabaee.d_at_hotmail.co.uk<mailto:pardis.tabaee.d_at_hotmail.co.uk>> wrote:
I think it's the average RMSD that was calculated. Maybe you can try to equilibrate without the solvent and see if you reach a stable conformation of your complex quicker than if you do it with the solvent? There are also other strategies. SMD is one of them, where you are applying constraints, you can do this with NAMD.
________________________________
From: owner-namd-l_at_ks.uiuc.edu<mailto:owner-namd-l_at_ks.uiuc.edu> <owner-namd-l_at_ks.uiuc.edu<mailto:owner-namd-l_at_ks.uiuc.edu>> on behalf of Radak, Brian K <bradak_at_anl.gov<mailto:bradak_at_anl.gov>>
Sent: 05 October 2016 17:18
To: namd-l_at_ks.uiuc.edu<mailto:namd-l_at_ks.uiuc.edu>; Roshan Shrestha; dhirajks_at_gmail.com<mailto:dhirajks_at_gmail.com>
Subject: RE: namd-l: question regarding rmsd
I'm not clear what the "problem" is here. Are you not happy with the (large?) oscillations after 100 ns? I don't understand why you would be surprised that convergence to a stable state takes that long. I'm more surprised that the apo state levels out in 20 ns.
I presume this is RMSD with respect to the initial frame, as is default for VMD? That's a completely arbitrary reference and thus I wouldn't hold too much stock in it. Is the initial bound state from a crystal structure or was it generated by docking to the apo structure? If the latter, then there isn't really a reason to believe the initial configuration had a high Boltzmann weight anyway - slow equilibration is to be expected and could involve large conformational changes.
Brian
Brian Radak
Postdoctoral Appointee
Leadership Computing Facility
Argonne National Laboratory
9700 South Cass Avenue, Bldg. 240
Argonne, IL 60439-4854
(630) 252-8643<tel:%28630%29%20252-8643>
brian.radak_at_anl.gov<mailto:brian.radak_at_anl.gov>
________________________________
From: owner-namd-l_at_ks.uiuc.edu<mailto:owner-namd-l_at_ks.uiuc.edu> [owner-namd-l_at_ks.uiuc.edu<mailto:owner-namd-l_at_ks.uiuc.edu>] on behalf of Roshan Shrestha [roshanpra_at_gmail.com<mailto:roshanpra_at_gmail.com>]
Sent: Wednesday, October 05, 2016 11:07 AM
To: namd-l_at_ks.uiuc.edu<mailto:namd-l_at_ks.uiuc.edu>; dhirajks_at_gmail.com<mailto:dhirajks_at_gmail.com>
Subject: Re: namd-l: question regarding rmsd
Don't worry dhiraj, it's obviously rmsd in Y-axis and Time steps in X-axis. Shouldn't have been any fuss with it !!!
On Wed, Oct 5, 2016 at 9:48 PM, <dhirajks_at_gmail.com<mailto:dhirajks_at_gmail.com>> wrote:
Sorry. I should have labeled it. Y axis is time in picosecond and x axis is rmsd in Angstrom.
Dhiraj
Sent from my iPhone
On Oct 5, 2016, at 10:55 AM, Pardis Tabaee <pardis.tabaee.d_at_hotmail.co.uk<mailto:pardis.tabaee.d_at_hotmail.co.uk>> wrote:
Hi,
What's on the y axis?
Regards,
P
________________________________
From: owner-namd-l_at_ks.uiuc.edu<mailto:owner-namd-l_at_ks.uiuc.edu> <owner-namd-l_at_ks.uiuc.edu<mailto:owner-namd-l_at_ks.uiuc.edu>> on behalf of Dhiraj Srivastava <dhirajks_at_gmail.com<mailto:dhirajks_at_gmail.com>>
Sent: 04 October 2016 22:07
To: namd-l_at_ks.uiuc.edu<mailto:namd-l_at_ks.uiuc.edu>
Subject: namd-l: question regarding rmsd
Hi
I am trying to do MD simulation on a protein with and without ligand. when I did rmsd plot, I found that apo protein is behaving fine (red) but ligand bound form (black) is taking relatively longer time to equilibrate and showing quite a bit of fluctuation in rmsd. is the fluctuation in rmsd value for ligand bound protein is acceptable or is there anything wrong? How can I fix it?
thanks
Dhiraj
[Inline image 3]
-- Roshan Shrestha Graduate Student Central Department of Physics,Tribhuvan University Kathmandu,Nepal

This archive was generated by hypermail 2.1.6 : Tue Dec 27 2016 - 23:22:31 CST