Highlights of our Work
2024 | 2023 | 2022 | 2021 | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 | 2014 | 2013 | 2012 | 2011 | 2010 | 2009 | 2008 | 2007 | 2006 | 2005 | 2004 | 2003 | 2002 | 2001
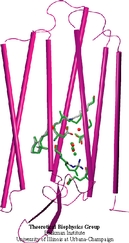
image size:
139.9KB
made with VMD
Resolving the physical processes that underly the biological function of a protein can be an elusive goal even with extremely detailed observations available. An example is the protein bacteriorhodopsin, a light driven proton pump in archaebacteria. This protein is a close relative to human G-protein coupled receptors that are the target for many pharmacological interventions and, hence, knowledge of bacteriorhodopsin's dynamics is of great medical interest. Despite the availability of highly resolved structures and spectroscopic observations of the protein and its functional intermediates, as they arise within 10-12 s of light absorption triggering its function, the physical mechanism remained ill understood. A recent computational modeling study that combined a quantum mechanical simulation of the protein's active site with a classical mechanical simulation of the remainder of the protein succeeded to fill in the elusive detail that reveals a complete picture of how the protein initiates proton pumping, a key step to explain entirely the biological function. For more information see here.



