HDL and Apo A-I Structure Prediction
 High density lipoprotein (HDL) circulates in the bloodstream, extracting cholesterol from body tissues and transporting it to the liver for excretion or recycling.
Increased levels of HDL have been correlated with a decreased risk of atherosclerosis---a primary cause of cardiovascular disease.
Nascent HDL particles are discoidal, consisting of a phosphatidylcholine bilayer and a protein shell which shields the hydrophobic lipid tails from the aqueous environment.
As it circulates in the body, HDL collects cholesterol which is then stored in the lipid bilayer.
Increased efficiency is achieved though the activation of the lecithin-cholesterol acyl transferase (LCAT) enzyme which converts the amphipathic cholesterol stored in the bilayer into hydrophobic cholesterol esters which collect among the lipid tails.
This induces a transformation of the HDL disk to a spherical form in which a hydrophobic core of cholesterol esters is shielded by a combination of lipid and protein.
At this stage cholesterol collection ceases and the mature HDL particle is recognized by the liver.
High density lipoprotein (HDL) circulates in the bloodstream, extracting cholesterol from body tissues and transporting it to the liver for excretion or recycling.
Increased levels of HDL have been correlated with a decreased risk of atherosclerosis---a primary cause of cardiovascular disease.
Nascent HDL particles are discoidal, consisting of a phosphatidylcholine bilayer and a protein shell which shields the hydrophobic lipid tails from the aqueous environment.
As it circulates in the body, HDL collects cholesterol which is then stored in the lipid bilayer.
Increased efficiency is achieved though the activation of the lecithin-cholesterol acyl transferase (LCAT) enzyme which converts the amphipathic cholesterol stored in the bilayer into hydrophobic cholesterol esters which collect among the lipid tails.
This induces a transformation of the HDL disk to a spherical form in which a hydrophobic core of cholesterol esters is shielded by a combination of lipid and protein.
At this stage cholesterol collection ceases and the mature HDL particle is recognized by the liver.
Apolipoprotein A-I (apoA-I) is the primary protein constituent of HDL, defining its size and shape, solubilizing its lipid components, removing cholesterol from peripheral cells, activating the LCAT enzyme, and delivering the resulting cholesterol esters to the liver. [POWN92] Because of the importance of HDL and the primary role of apoA-I in its function, there is a great deal of interest in the lipid-bound structure of apoA-I. Native HDL consists of heterogeneous particles with possibly diverse conformations of apoA-I, precluding structural studies of apoA-I in this form. Fortunately, there exists a general method for the creation of reconstituted HDL (rHDL) particles with a specified phospholipid and apolipoprotein content which are analogous to native nascent HDL, displaying all of its physiological functions. [JONA86B] The use of nondenaturing gradient gel electrophoresis has led to the discovery of discrete rHDL species of defined size and composition. The diameters of these rHDL disks vary with the number of apoA-I molecules per particle (2, 3, or 4) and their conformation. [HEFE90a,JONA89]
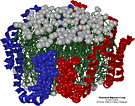
In a collaboration with Professor Ana Jonas of the College of Medicine of the University of Illinois (UIUC), who performs experiments on reconsituted HDL, researchers combined experimental evidence on the structure of the HDL particle with methods for predicting protein structure from sequence data to produce a preliminary model of the HDL particle. This model surrounded a circular disk of 160 lipids, forming a bilayer, with two apoA-I proteins and 6000 water molecules, altogether a 46,000 atom system. The system was refined via 250 ps of simulated annealing with NAMD on the Resource's local four-processor HP servers. The resulting HDL particle was found to be stable in molecular dynamics simulations and it was concluded that a plausible model for the apoA-I structure had been obtained. [PHIL97]
Personnel
- James Phillips 1 --- Structure prediction and molecular dynamics simulation.
- Willy Wriggers 1 --- Molecular dynamics simulation.
- Zhigang Li 1 --- Structure prediction.
- Ana Jonas 2 --- Experimental studies of rHDL.
1 Theoretical Biophysics, Beckman Institute, and Department of Physics.
2 Department of Biochemistry, College of Medicine at Urbana-Champaign.
Publications
James C. Phillips, Willy Wriggers, Zhigang Li, Ana Jonas, and Klaus Schulten. Predicting the structure of apolipoprotein A-I in reconstituted high density lipoprotein disks. Biophysical Journal, 73:2337--2346, 1997. [PHIL97]
All figures created with VMD.



