Highlights of our Work
2024 | 2023 | 2022 | 2021 | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 | 2014 | 2013 | 2012 | 2011 | 2010 | 2009 | 2008 | 2007 | 2006 | 2005 | 2004 | 2003 | 2002 | 2001
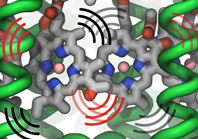
image size:
231.6KB
made with VMD
Photosynthetic life forms bottle the energy of sun light. How do they
do it? Fast! Indeed, the first nanosecond after absorption of sun
light is crucial in photosynthetic light harvesting. During this time
absorbed solar energy is in its least stable form, that of
electronically excited molecules which decay by re-emitting a photon
(fluorescence) at a rate of 1 every nanosecond (1 every 0.000000001
seconds). Fluorescence would be wasteful to the organism and to avoid it
the molecular excitation energy is transported over tens to hundreds of
nanometers through an energy transfer network to so-called
photosynthetic reaction centers where it is converted into a more stable
form of energy (see our recent review on light harvesting). The fast
transport is achieved by transferring excitation energy between clusters
of strongly interacting pigment molecules that act as stepping stones
and as a result the excitation energy is used in about 0.1 nanoseconds,
i.e., within 10% of the fluorescence decay time, thus bottling sun light with an
efficiency of 90%. The thermal motion of the pigment molecules and their
protein scaffold greatly influences the excitation transport. A recent study
showed that correlated thermal fluctuations that arise in pigment
clusters affect the excitation transfer particularly strongly, typically
slowing transfer it down. Pigment clusters that avoid correlated thermal
motion increase the efficiency of light harvesting. More information can
be found here.



